INTRODUCTION
Korean C. japonica is one of ecologically significant portunid crab species in the Yellow Sea, belonging to the family Portunidae, and the order Decapoda. In the environmental ecosystem, Charybdis crab is broadly inhabited in the seawater areas of the Korean peninsula, besides in some regions in the East China Sea, Taiwan, Malaysia and Hawaii. Apparently, the dorsal surface of shell is habitually smooth. The crab has very large and long both claws and the body color of this crab is light grey form. Since crabs are night-time organisms, they nestle under the grits, sandy soil and rocks of the shallow sea. Many explores have displayed that the change of water temperature by earth warmth, population density, tide embankment, thoughtless overhunting and environmental pollution are serious in the early larval development of this portunid crab (Yeon et al., 2011). Charybdis crab is one of commercial portunid crab in Korea all the year round.
However, notwithstanding their economic and ecological assessments, only a few data presently are existent, which are acknowledged as environmentally (Yeon et al., 2011), sitologically (Ho, 2001; Oh, 2002; Park et al., 2008), ecologically (Seo & Hong, 2009), morphologically (Heo et al., 2006), and behaviorally (Kim & Ko, 1987) compared to other crayfish species. Thus, there is a requirement to understand the genetic characters and more statistics of this crab in order to evaluate exactly the accurately genetic suggestion. Above all, the clustering analysis of the genetic distance (GD) between populations/species/genera of several teleost and crustaceans from the different geographic sites has been accomplished by means of PCR technique is a few quantity (Diaz-Jaimes & Uribe-Alcocer, 2003; Wang & Li, 2004; Wasko et al., 2004; Nagarajan et al., 2006; Upadhyay et al., 2006; Ghatak et al., 2007; Yoon et al., 2007; Kang & Yoon, 2013; Yoon, 2020; Jo & Yoon, 2021).
This scrutiny tries to illuminate the GDs and polymorphism within and between Charybdis crab collections. With the aim of accomplishment, this author achieved clustering analyses of Korean Charybdis crab (C. japonica) in the Yellow Sea of Korea.
MATERIALS AND METHODS
Two assemblies of Charybdis crab (C. japonica) were taken from Seosan in the vicinity of the Yellow Sea of Korea. Two sample collections of crab muscle was gathered in disinfected cylinders, proximately positioned on cold ice, and retained at −79°C until required. PCR inquiry was achieved the genomic DNAs from 22 individuals, consuming different five oligonucleotides primers (ONT-primers). The extraction/refinement of genomic DNA (gDNA) was accomplished under the requirements described previously (Yoon, 2018). Proteinase K solution was involved to the tubes and gradually pipetted for some minutes.
Five ONT-primers (Operon Technologies, Alameda, CA, USA) was between 60%–70%. OPA-16 (5’-AGCCAGCGAA-3’), OPB-10 (5’-CTGCTGGGAC-3’), OPB-15 (5’-GGAGGGTGTT-3’), OPD-10 (5’-GGTCTACACC-3’), and OPD-20 (5’-ACCCGGTCAC-3’) were the primers spent to classify the unique loci shared to each crab population (ULSECP) and the amount of loci shared by the two crab populations (LSTCP) was estimated. PCR examination was executed on a recorded genomic thermal cycler (MJ Research, Waltham, MA, USA). DNA increase was executed with 27 μL example cylinders comprising 5 ng of template DNA, 20 μL of premix (Bioneer, Daejeon, Korea), and 2 unit of primer. PCR outcomes of the increase feedback were distributed by electrophoresis for 30 min at 100 V in a 1.4% agarose gel, tainted with EtBr and pictured under ultra-violet beam, and took pictures of on a transilluminator using a gel evidence apparatus (PECA Products, Beloit, WI, USA).
Similarity matrixs (SMs) were established based on the data of bandsharing (BS) rates constructed by molecular analysis. Comparing the two lanes, the BS rate was appraised as surveys: BS = 2 (Nab) / (Na + Nb), where Nab signifies the amount of FMs shared by examples b and a; Na points out the total quantity of FMs in a; and Nb signifies the total quantity of FMs in example b. The median within-group correspondence was rated via pairwise matching inquiry between the parties within a group. A polar hierarchical dendrogram (PHD) was generated renowned on SMs to take a cluster tree using Systat version 10 (SPSS, Chicago, IL, USA).
RESULTS AND DISCUSSION
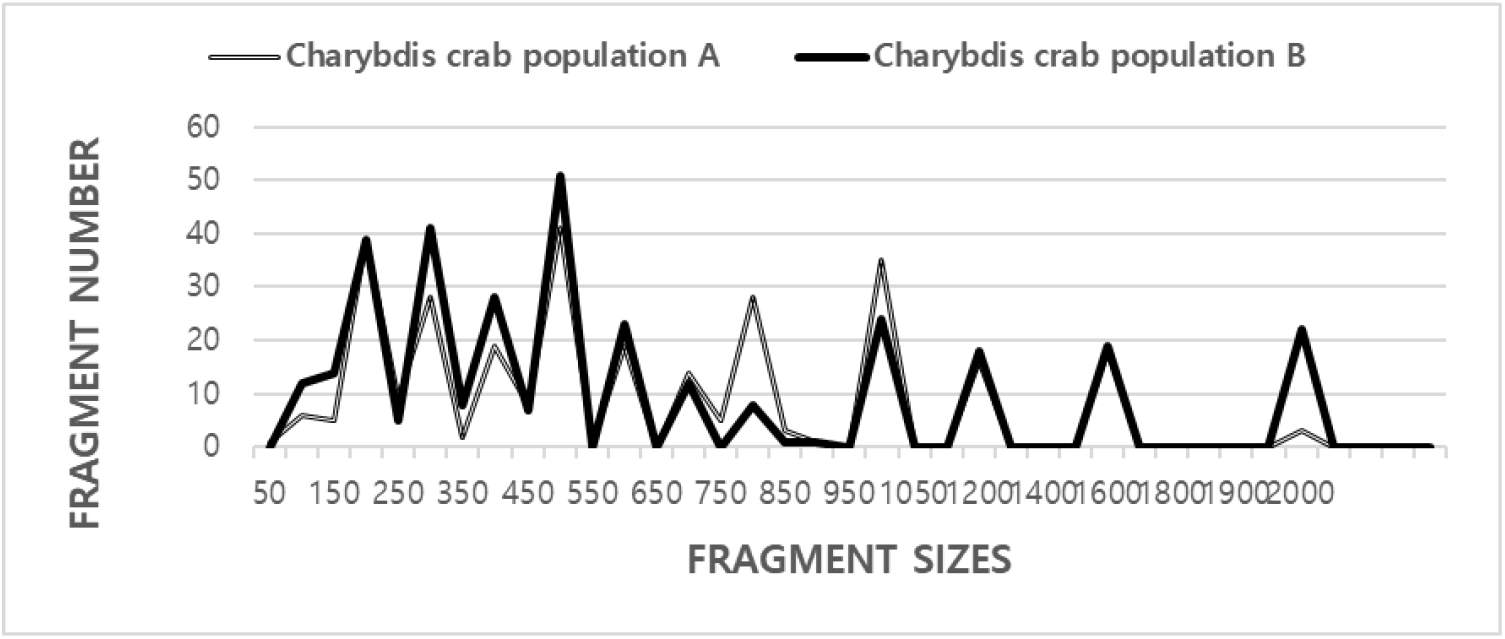
GDNA set apart from two populations of Korean Charybdis crab (C. japonica) was augmented some rounds by PCR experiments. The fragment (FRAG) amounts in each size interval have been computed from the integrated FRAGs obtained with every five ONT-primers. The higher FRAG extents (>1,900 bp) are perceived in the Charybdis crab population A (CCPA), as shown in Fig. 1. Seven ONT-primers produced a sum of 884 FRAGs in the black rockfish species and 632 in the Hwanghae rockfish species, with a DNA FRAGs amount stretching from 150 to 2,200 bp (Yoon et al., 2007). The extents of the DNA FRAGs also varied roughly, from 90 to 2,400 bp in Gracilaria vermiculophylla and G. chorda (Kim & Yoon, 2018). Yoon (2018) also described that the more FRAG amounts (>1,100 bp) are not identified in di-population of the razor shellfish (Solen corneus). The five ONT-primers created a whole of 367 FRAGs in the innate pufferfish population and 211 FRAGs in the farmed pufferfish population, with genetic FRAG extents fluctuating from 50 bp to 1,300 bp (Yoon, 2020). The mean quantity of separate FRAGs per primer within the innate and cultivated river pufferfish population was 14.68 and 8.44, individually (Yoon, 2020). The number of the FRAGs showed 354 and 390 DNA countable FRAGs, respectively, extending from 100 bp to 1,600 bp for the Yeosu and Jinhae populations of the arkshell species (Yoon, 2021a).
The BS value between individual’s no. 01 and no. 10 was the lowest (0.371) between the two CCPs and the value between individual’s no. 10 and no. 11 was the highest (0.818), as shown in Table 1. The five ONT-primers, OPA-16, OPB-10, OPB-15, OPD-10, and OPD-20 were spent to yield the number of ULSECP and number of LSTCP (Table 2). 305 FRAGs were identified in the CCPA, and 344 in the Charybdis crab population B (CCPB): 44 number of ULSECP (14.43%) in the CCPA and 110 (31.98%) in the CCPB. 44 number of LSTCP, with an average of 8.8 per primer, were detected in the two crab populations. The ONT-primer OPD-20 generated 44 identical DNA FRGMs, nearly 200 bp and 300 bp, in both the CCPA and CCPB. Assessed independently, the average molecular difference (MD) was higher in the CCPB than in the CCPA.
The ONT-primer OPC-07 created 44 unique loci shared to each pufferfish population (ULSEPP) of the refined river pufferfish (Yoon, 2020). The author contended that the ONT-primer OPC-05 discovered 44 loci shared by all samples of the two river pufferfish populations, as thick and/or thin FRAGs of 500 bp and 1,200 bp, separately. Also, the author stated that five ONT-polymers were expended making a full of 110 LUECP in first group and 132 in second group in two Scapharca subcrenata populations, separately, shifting in amount of DNA FRAGs from larger than nearly 50 to below 1,050 bp (Yoon, 2021b). As prepared reference of clams, shrimps and oysters, for PCR scrutiny, Yoon & Kim (2003a) stated that 7 ONT-primers created 585 thick and thin DNA FRAGs from three terrestrial locations, making roughly 6.6 median yields per ONT-primer in marsh clams (Corbicula spp.) from Gochang. McCormack et al. (2000) contended that DNA FRAGs acquired by four ONT-primers ranged from 100 to 2,300 bp in the brittle star (Amphiura filiformis). It has been informed that 7 ONT-primers produced 317 FRAGs in a farmed shrimp population, and 385 in the untamed population, extending from 100 to 1,800 bp (Yoon & Kim, 2003b).
SM, similarity matrix; BS values, bandsharing values; CCPs, Charybdis crab populations; CCPA, Charybdis crab population A; CCPB, Charybdis crab population B.
ULSECP, unique loci shared to each crab population; LSTCP, loci shared by the two crab populations.
CCPs, Charybdis crab populations; ONT-primers, oligonucleotides primers; CCPA, Charybdis crab population A; CCPB, Charybdis crab population B; FRAGs, fragments.
The average bandsharing values (ABS values) was 0.457±0.007 between the CCPA and CCPB, as established in Table 3. The ABS values of individuals in the CCPA (0.575±0.014) were lesser than in those originated from the CCPB (0.705±0.011) (p < 0.05). To elucidate, reports have exposed that the median BS value attained expending five ONT-primers was 0.852 in the rainbow trout population, 0.704 in the masu salmon population (Yoon, 2020), and 0.282±0.008 between the two terrestrial oyster populations (Kim et al., 2004).
The PHD achieved by the five ONT-primers denotes three genetic clusters (GCs): cluster I (CHARYBCRAB 01, 04, 05, 06, and 08), cluster II (CHARYBCRAB 02, 03, 07, 09, 10, and 11) and cluster III (CHARYBCRAB 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, and 22), as shown in Fig. 2. The shortest GD displaying significant MD was between individuals CHARYBCRAB no. 18 and CHARYBCRAB no. 17 (0.055). The longest GD showing significant MDs between two Charybdis crab populations was observed in individuals CHARYBCRAB no. 06 and CHARYBCRAB no. 01 (0.632). In due course, individual no. 01 of the CCPA was most distantly related to CCPA no. 06 (GD=0.632).
| Population | CCPA | CCPB |
|---|---|---|
| CCPA | 0.575±0.014 b | 0.457±0.007 c |
| CCPB | – | 0.705±0.011 a |
Each assessment is an outcome of three unlike tests.
ABS values, average bandsharing values; BS values, bandsharing values; SM, similarity matrix; CCPA, Charybdis crab population A; CCPB, Charybdis crab population B.
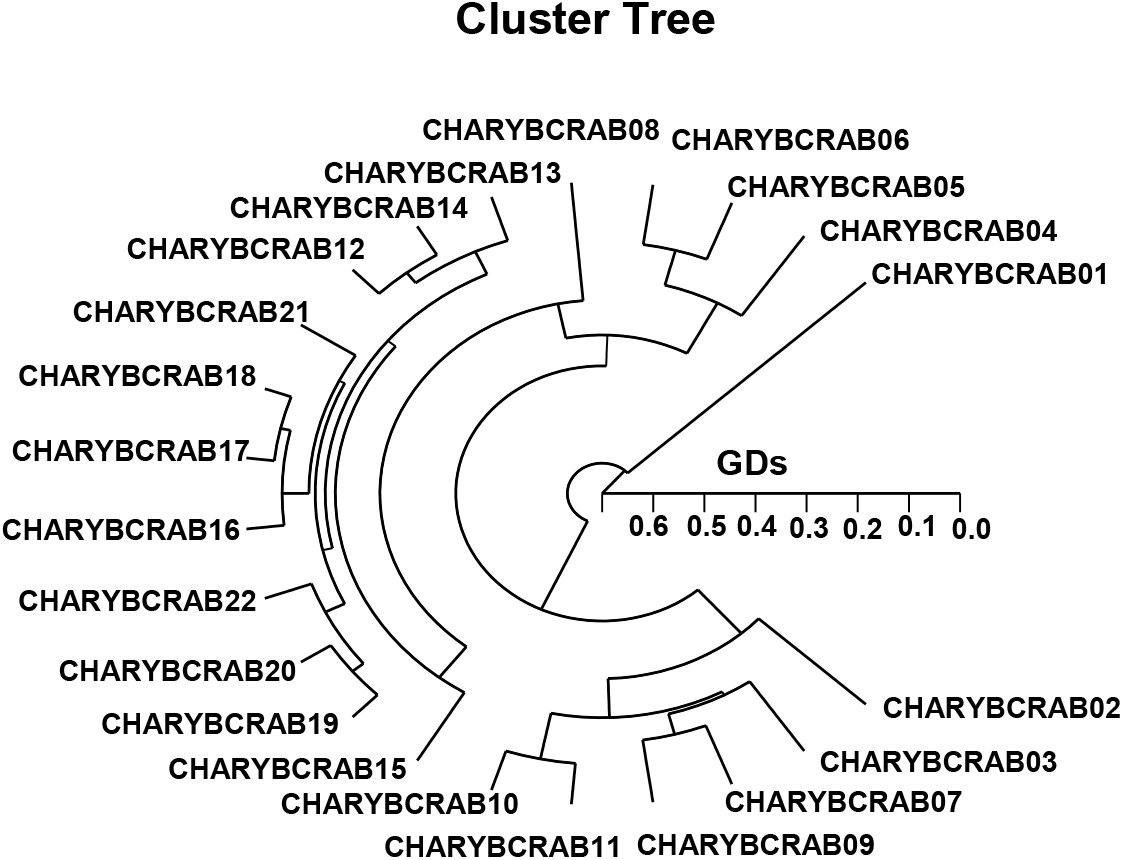
In finfish, this grouping scrutiny acquired a form consistent with the one postulated by Nagarajan et al. (2006). Amid the three Channa punctatus collections gathered from a few waterways of south India, the maximum hereditary space (GD=0.9231) was acquired between two sample collections (Nagarajan et al., 2006). This analysis presented that great MDs could be achieved among individuals of terrestrial sample assembles. The rates of the pairwise appraisals of balanced GD between the populations of the tailfin anchovy (Coilia nasus) from the pooled records for the seven ONT-primers, ranged from 0.051 to 0.435 (Jo & Yoon, 2021). The GD between individuals thus confirmed the reality of a neighboring affiliation in group II between two populations of tailfin anchovy. As mentioned formerly, the promise of genomic PCR to ascertain analytical markers for the recognition of the two CCPs has been validated. S. subcrenata bi-group can be obviously categorized by gDNA-grounded processes.
Numerous investigators scrutinized the measures of DNA FRAGs in the genomic products of yellowfin tuna (Thunnus albacares) (Diaz-Jaimes & Uribe-Alcocer, 2003), Amazonian fish matrincha (Brycon cephalus) (Wasko et al., 2004), Chryseobacterium strains (Bernardet et al., 2005), spotted murrel (C. punctatus) (Nagarajan et al., 2006), crayfish (Cambaroides similis) (Kim et al., 2004), yellow grouper (Epinephelus awoara) (Upadhyay et al., 2006), Aeromonas spp. (Ghatak et al., 2007), rockfish (Sebastes spp.) (Yoon et al., 2007), gracilaria (Gracilaria vermiculophylla) (Yoon, 2018), river pufferfish (Takifugu obscurus) (Yoon, 2020), and tailfin anchovy (C. nasus) (Jo & Yoon, 2021). The apparent primer revealed significant MDs in parties and groups, resulting from variations in DNA polymorphisms among parties and groups (Archak et al., 2003; Diaz-Jaimes & Uribe-Alcocer, 2003; Wang & Li, 2004; Wasko et al., 2004; Kim et al., 2006; Nagarajan et al., 2006; Yoon, 2018; Yoon, 2020; Jo & Yoon, 2021).
Above-mentioned cluster analysis exposed an association between the individuals within two crustacean assemblies relatively declaring added invertebrates (Yoon, 2018). In the other clams, cluster analysis of the pairwise group matrix, produced from inherited identifications, designated that geographically close clusters be arranged to collect jointly in the blacklip abalone (Huang et al., 2000). The ability of ONT-polymers enlarged DNAs to disclose distinctive markers for breed, stock, species, genus, and assembly evidence of personality in life individuals (Esselman et al., 2000; Huang et al., 2000; Dixon et al., 2004; García et al., 2004; Araneda et al., 2005; Gelin & Souty-Grosset, 2006; Godhe et al., 2006; Upadhyay et al., 2006; Ghatak et al., 2007; Yoon et al., 2007; Kang & Yoon, 2013; Kim & Yoon, 2018; Yoon, 2021b) has also been fine accepted.
Great point of an important GD between two S. subcrenata groups showed this research process is one of the most appropriate apparatuses for biotechnological DNA studies on entities and units of other life beings (Koh et al., 1998; Dixon et al., 2004; Araneda et al., 2005; Godhe et al., 2006; Kang & Yoon, 2013; Yoon, 2018). It has been described that the ONT-primer was valuable in the documentation of entities and/or groups, resulting from variations in DNA polymorphisms among entities/populations (Archak et al., 2003; Diaz-Jaimes & Uribe-Alcocer, 2003; Yoon & Kim, 2003a; Wang & Li, 2004; Wasko et al., 2004; Kim et al., 2006; Nagarajan et al., 2006; Upadhyay et al., 2006; Ghatak et al., 2007; Yoon et al., 2007; Kang & Yoon, 2013; Yoon, 2020; Jo & Yoon, 2021; Yoon, 2021a).

