INTRODUCTION
Acetaminophen [Paracetamol, N-acetyl-para-aminophenol (APAP)] is a common over-the-counter analgesic agent. As nonsteroidal anti-inflammatory drugs (NSAIDs) such as acetylsalicylic acid (aspirin) and ibuprofen (IBU), acetaminophen is widely used to treat inflammation and pain (Kristensen et al., 2016).
Acetaminophen has many functions of reducing pain, reducing fever, and relieving the symptoms of allergies, cold, cough, headache, and influenza. A commercial brand that contains the acetaminophen as an active ingredient is Tylenol, whose brand name is derived from a chemical name for the compound, N-acetyl-para-aminophenol (Nancy, 2014). That is, the last three letters of N-acetyl and last four letters of aminophenol were combined in the brand.
It is safe at therapeutic doses for analgesic and antipyretic therapy (Jóźwiak-Bebenista & Nowak, 2014). But overdoses or the long-term use of acetaminophen resulted in toxicity effects in the organs of liver, kidney, and testis and affected the blood chemistry and reproductive parameters (Olaleye & Rocha João, 2008; Luangpirom et al., 2012; Oyedeji et al., 2013; Khayyat, 2021). High doses of acetaminophen affected the male reproductive system, changing the semen quality, particularly the sperm morphology and fertilizing ability (Ratnasooriya & Jayakody, 2000; Luangpirom et al., 2012; Oyedeji et al., 2013; Banihani, 2018; Boizet-Bonhoure et al., 2022).
Acetaminophen influenced the semen quality by reducing testosterone synthesis, inducing oxidative stress, provoking the apoptosis of spermatocytes, suppressing nitric oxide production, and inhibiting prostaglandin synthesis (Banihani, 2018). Many studies showed that overdoses and long- term acetaminophen increased the risk of oxidative stress, damaging hepatocytes, renal tubules, testicular tubules, and blood cells, and causing hypertension and heart infarction (Olaleye & Rocha João, 2008; Luangpirom et al., 2012; Oyedeji et al., 2013; Banihani, 2018; Khayyat, 2021).
Recently, the effects of acetaminophen and NSAIDs on filial generation and on reproductive parameters have been reviewed (Boizet-Bonhoure et al., 2022; Tadokoro-Cuccaro et al., 2022). Generally exposure to NSAIDs was associated with the negative effects on the reproductive track development and function in prenatal and adult rodents.
The long-term treatment with acetaminophen at high dose (500–1,000 mg/kg for 30 days) impaired sexual competence, sperm count and quality, and fertility in adult rat (Ratnasooriya & Jayakody, 2000). Those effects were reversible. Thus, the anti-reproductive influence was not general toxic effects but induced an increase of pre-implantation losses due to oligozoospermia, impairments of sperm motility, and reduction of potency of fertilizing spermatozoa (Ratnasooriya & Jayakody, 2000).
Spermatogenesis is the dynamic process by which spermatozoa (sperm) are produced from spermatogonium cells in the seminiferous tubules of the testis (de Kretser et al., 1998). In many small mammals inhabiting in temperate zone reproductive activities present a characteristic of seasonal breeding, showing active and big testicles in summer and inactive and small testicles in winter, which are discernible by naked eyes. They show a cyclic alteration of active and inactive spermatogenesis (Stetson & Watson-Whitmyre, 1984, 1986; Choi & Lee, 2012; Choi, 2019; Jeon et al., 2020). Their spermatogenesis is arrested in the winter season due to the shortened length of day time called short photoperiod (SP). And animals are unable to breed for several months. The seminiferous tubules of the regressed testis contain only Sertoli, spermatogonial cells, and initial stage cells of spermatogenesis, displaying arrest of meiosis process. Whether the testicular regressing process by SP is same as the testicular regressing process by the treatment of acetaminophen is needed to be examined.
Therefore, the goal of the present work was to examine the chronic effects of acetaminophen in testicles of the golden hamsters who received various doses of acetaminophen in diet. Also, the effects of acetaminophen on testicles were compared to those of SP that was on the way of reproductive regressing course.
MATERIALS AND METHODS
Mature male golden hamsters (Syrian hamsters, Mesocricetus auratus) were used in this investigation. They were divided into 5 groups and subjected to this experiment for 4 weeks: animals housed in long photoperiod (LP, lights of 14 hours and darkness of 10 hours in a day) as LP control, animals housed in SP (lights of 10 hours and darkness of 14 hours in a day) for 4 weeks as SP control (SP4), and groups of animals treated with low, middle, and high concentrations of acetaminophen (Low, Middle, High groups). Also animals housed in SP for 8 weeks were included (SP8) to contrast the testicular activities, if necessary.
The animals were kept in animal facility and the lighting scheme was strictly regulated by the plug-in timer as the their reproductive features are regulated by photoperiod. LP maintains the reproductive activity energetically but SP induces testicular regression in 8 weeks in an artificial lighting regimes of the animal facility. The animals were housed in boxes which were made by the wooden board. The small fan was equipped in one side of the box. The outer lighting was blocked completely by using dangling black wrappers. Sanitary conditions were periodically managed. The ambient temperature was set at 22±1°C. The condition of management of animals was approved by the Yongin University Institutional Animal Care and Use Committee (YUIACUC-2020-02).
As the solubility of the acetaminophen (A7085, Sigma-Aldrich, St. Louis, MO, USA) in water, alcohol, and other solvents is low, the diet was reconstructed at each concentration. High, middle and low concentration of the diets contained 15,000 (15 mg/g of diet pellet), 3,000, and 600 ppm of acetaminophen, respectively. The animals were fed with standard laboratory mouse chow or reconstructd diets containing different concentrations of acetaminophen. The quantities the animals fed were measured at a given time every next day. They were supplied with tap water ad libitum. Mature animals were maintained in LP or transferred to SP. They were housed in each photoperiod for 4 full weeks. Another group of animals were housed in SP for 8 weeks.
The amounts that animals fed diet were determined in evey morning. The quantity of feeding diet in a specific day was calculated as an amount subtracted the remained diet from the supplied amount of diet in a given time of every day. To account for the variation in the amount of diet consumed by individual animals, the average daily amounts of diet consumed by each group for a week were calculated and plotted.
The body weights of hamsters were measured at initial and final time to examine any fluctuation. The testicular volume was measured by laparotomy using vernier calipers (series 530, Mitutoyo America, Aurora, IL, USA) at the beginning and the end of 4 full weeks. The animals were briefly anesthetized with diethyl ether. Following the excising the skin overlying the scrotal sac and protruding the testicles within the scrotal sac, the major axis and the minor axis of the testicles were immediately measured by vernier calipers. The skin excised was sutured with autoclips (CLAY ADAMS® brand, MikRon Precision, Monroe, CT, USA). At the end of the experiment, after the determination of the testicular mass as mentioned above, the animals were decapitated and the various internal organs, including the reproductive accessory organs, were isolated and directly weighed. The testicles were kept in formalin until use for histological examination.
The measured values of the testicles were converted into testicular mass by calculating the major axis and the minor axis via a converting equation develped previously (Watson-Whitmyre & Stetson, 1985). The calculated masses of testicles were compared, plotted, and analyzed with the testicular weights those were directly weighed.
The paraffin tissue sections were applied for the histological examinations of testicles. The tissue samples were processed according to standard histology procedures, being fixed in formalin, dehydrated through alcohol and xylene passages, and embedded in a block of paraffin wax for slicing by a microtome (5 μm). Tissues were deparaffinized with xylene, rehydrated with ethanol, and stained. The slices were mounted on slide glasses and the slides were subjected to hematoxylin- eosin stain, respectively. They were left for a while to evaporate in the air and treated with Canada balsam (Duksan Pure Chemicals, Ansan, Korea) for permanent specimen, and observed under microscope (Leica DM500).
According to the distribution of germ cells in histological view of testicles, the seminiferous tubules in each group simply assorted into three ranges: full spermatogeic tubules designated by active, completely arrested spermatogenic tubules designated by inactive, and the tubules that germ cells were absconded from the original location, designated by drift.
RESULTS
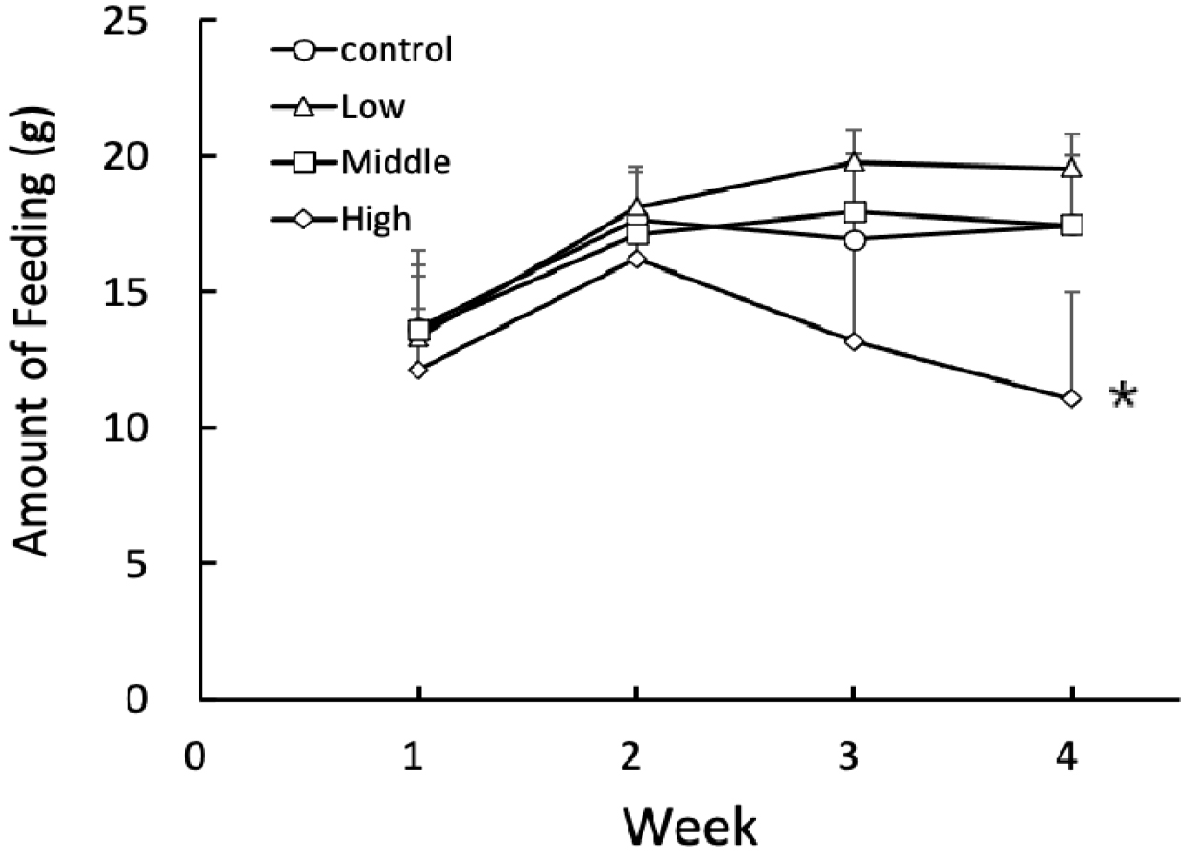
The animals increased the amount of feeding diet for 2 weeks from the beginning of the present experiment (Fig. 1). Afterward the amounts of feeding diet were not altered severely in the control, low, and middle groups except for the high group. In the high group, the amounts of feeding diet for the continuing 2 weeks were significantly (p<0.05) reduced in comparison to the all other groups. But there was no any particular aberrant behavior in any animal groups.
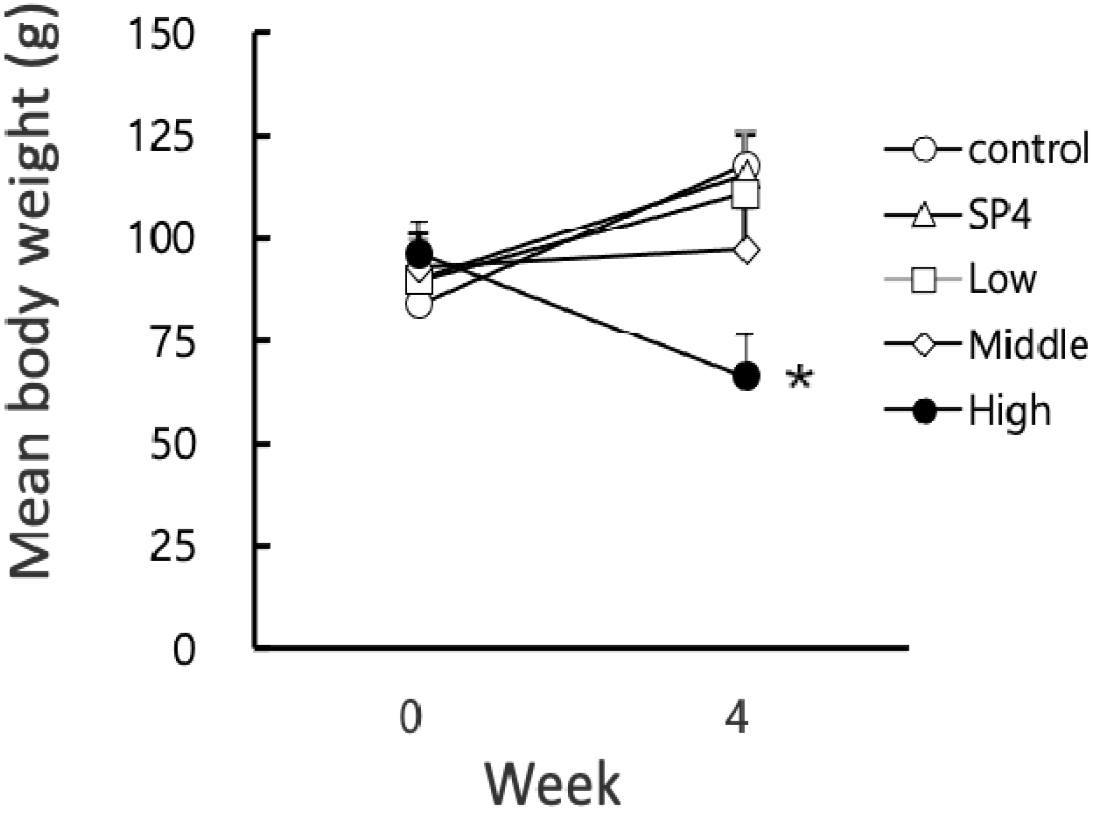
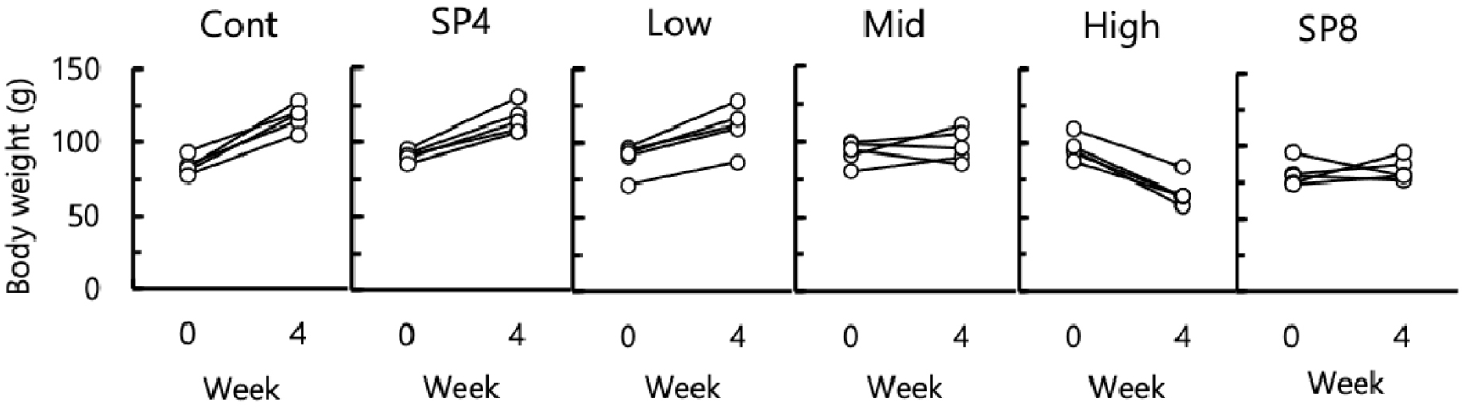
The body weights of all animals were weighed at both initial and final times of the experiment. The mean body weights of animals also increased all the time from the beginning of the present study with a different degree except for the high group (Fig. 2). The mean body weights of the animals in high group was significantly (p<0.05) reduced at the end of the experiment in comparison to all other groups. The changes of body weight in individual animals was indicated in Fig. 3. The individual animals showed an increase of body weight in LP control, SP4, and low groups. In the middle group, three animals had an increase of body weights and two animals had a decrease of body weights. In high group, all the animals exhibited a decrease of body weights. The changes of body weights were compatible to the quantity of feeding diet.
Various internal organs were actually isolated and weighed to inspect any weighable alterations at the end of experiment (Table 1). Heart, lungs, and spleen were not changed in weights among the groups. But the weights of liver and kidney were significantly dimished in high group (p<0.05).
Data are represented as the mean±SD (n=5).
* Indicates statistical significance compared to other groups (p<0.05).
Cont, animals housed in LP condition; SP4, animals housed in SP condition for 4 weeks; Low, Middle, and High, animals housed in LP condition and fed food reconstructed in low, middle, and high concentration of acetaminophen in diet, respectively; SP8, animals housed in SP condition for 8 weeks; LP, long photoperiod; SP, short photoperiod.
The actual weights of reproductive organs, including testicles, epididymides, and seminal vesicles, were lowered significantly (p<0.05) in comparison to all other groups (Table 1 & Fig. 4).
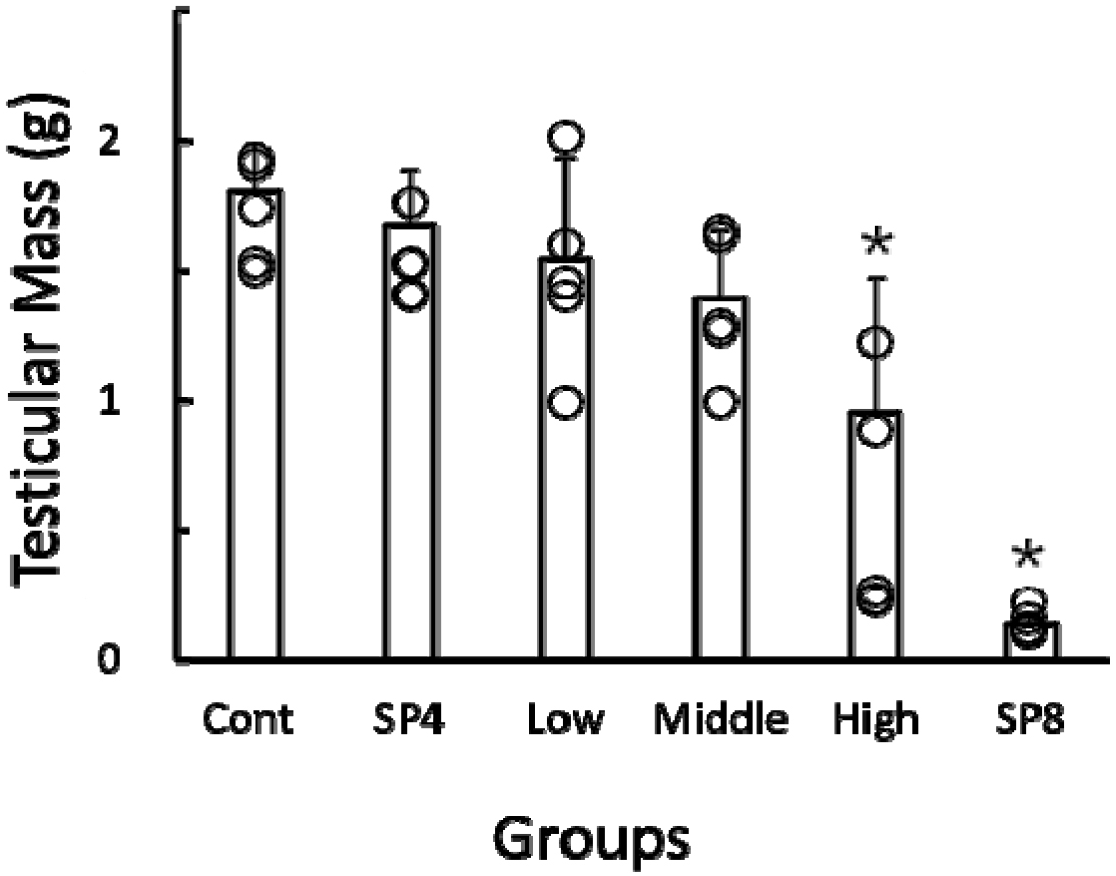
By using the values of both the major axis and the minor axis of the testicles, the testicular masses were calculated and were employed on behalf of the actual testicular weights as reported previously (Watson-Whitmyre & Stetson, 1985). The testicular masses were increased in all groups except for high group (Fig. 5). The only high group showed significantly (p<0.05) reduced testicular masses at the end of this experiment in comparison to all other groups. All the animals in SP4 group presented apparently undiminished testicles and unchanged reproductive accessory organs. The animals housed in SP for 8 weeks (SP8) were included for reference and displayed distinctly regressed testicles and reduced accessory organs.
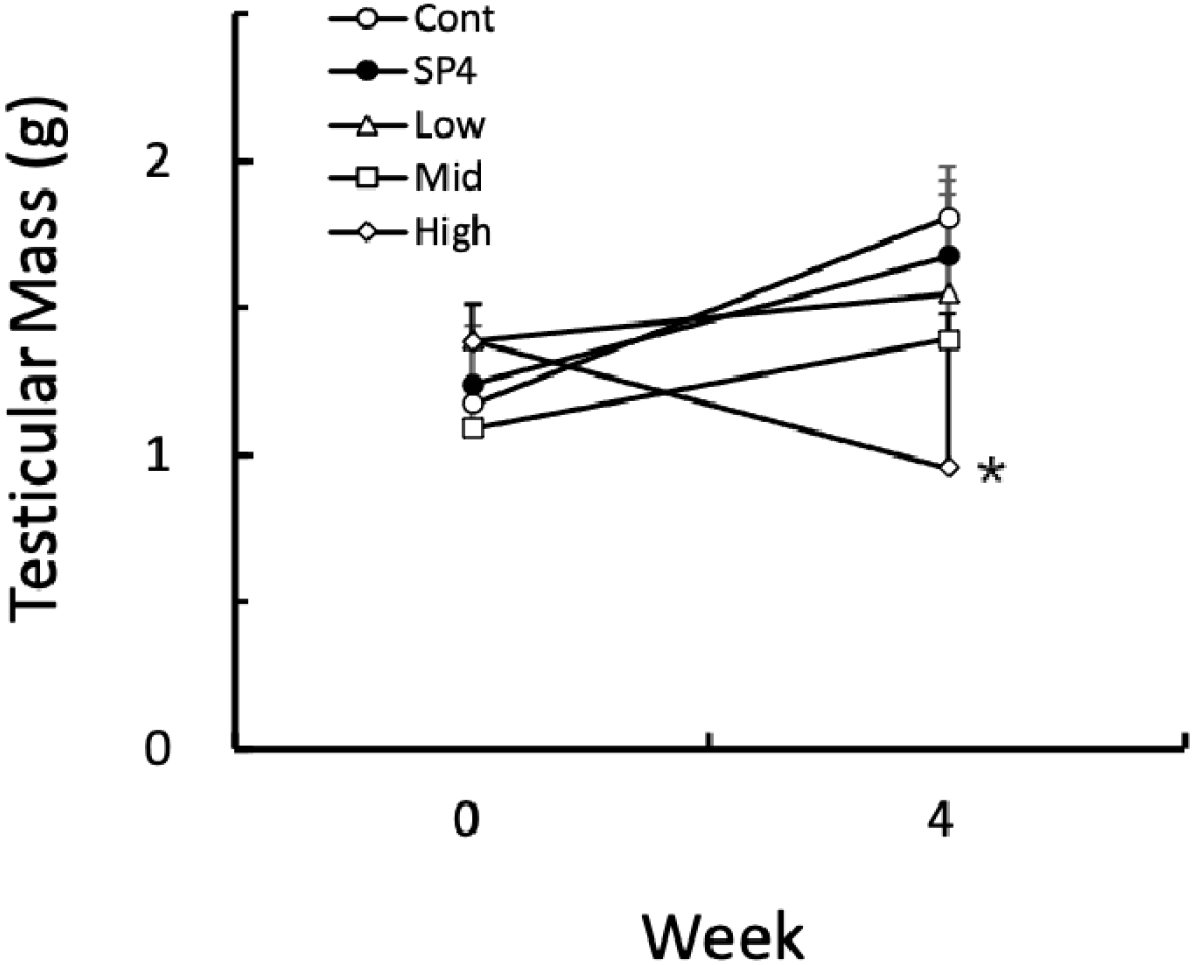
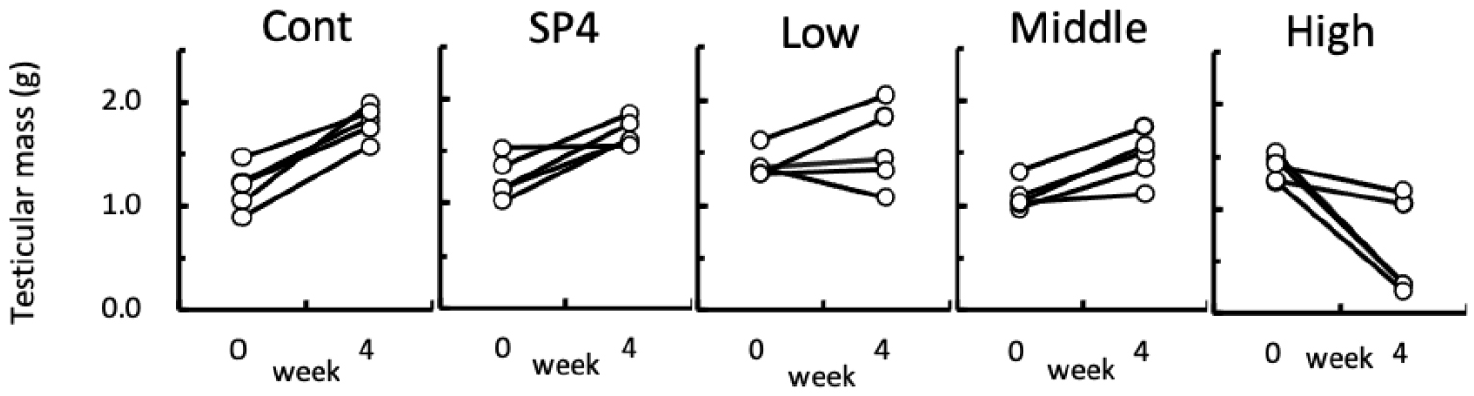
Fig. 6 shows the individual changes of testicular masses of hamsters in each group. At the beginning of this experiment all animals had the large and active testicles. At the 4th week of treatment, all groups except for high group still showed relatively large testicles although the testicles became larger with a various degree, still sustaining the functional spermatogenesis. The animals treated with high concentration of acetaminophen showed size differences with the naked eye, which were large or tiny testicles (Fig. 6), demonstrating completely discernible reproductive aspects. As indicated in Fig. 6, two animals still had massive and big testicles and the remaining three animals showed very small testicles, indicative of sexually active and inactive spermatogenesis, respectively. In the histological views of testicles the former renamed as high A and the latter high B (Fig. 7).
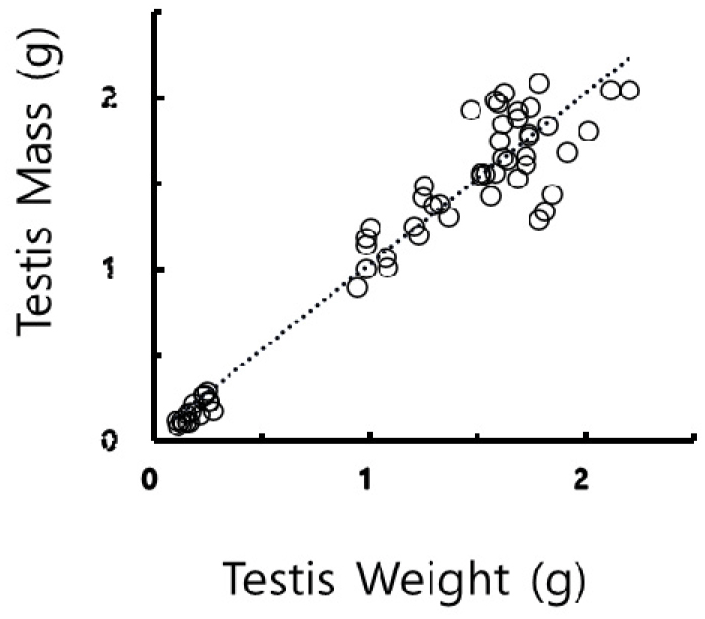
The mass values determined by the calculation were compared to the actual testicular weights values weighed by direct weighing and examined for the mutual correlation between the masses and the weights. The correlation coefficient resulted in 0.93, as showed in Fig. 8. The correlation could be evaluated that the values converted from the measures exhibited the values weighed literally as reported previously (Lee et al., 2013; Jeon et al., 2020). The outcome implies that the values calculated could completely replace substantially the values weighed directly.
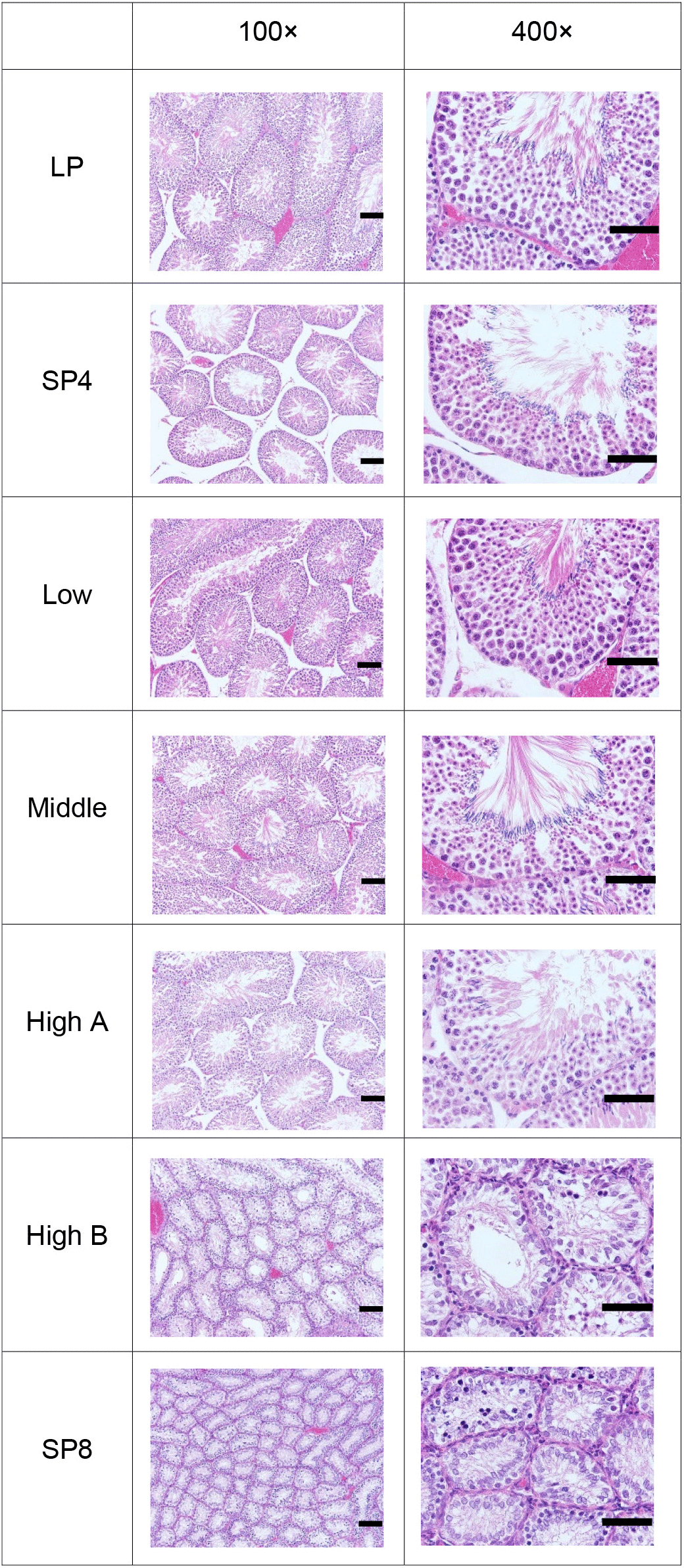
The active and full spermatogenic testicles, including spermatogonia, spermatocytes, spermatids, and spermatozoa with tails were observed in LP control, SP4, low, middle groups, and high A groups (Fig. 7). Contrarily the completely regressed and inactive testicles were shown in high B and SP8 groups. The animals in high B group had primarily spermatogonia and some spermatocytes without any spermatids in the epithelium of the seminiferous tubules and no spermatozoa in the lumen of the tubules. These results were apparent in the spacious diameter of the seminiferous tubules. The average diameter of the tubules of active testicles was near 300 μm. The diameter of the seminiferous tubules in regressed testicles was roughly less than half in length, meaning approximately one eighth in volume compared to the active testicles. Some germ cells were naturally or experimentally dislocated from the original epithelial layer of the seminiferous tubules.
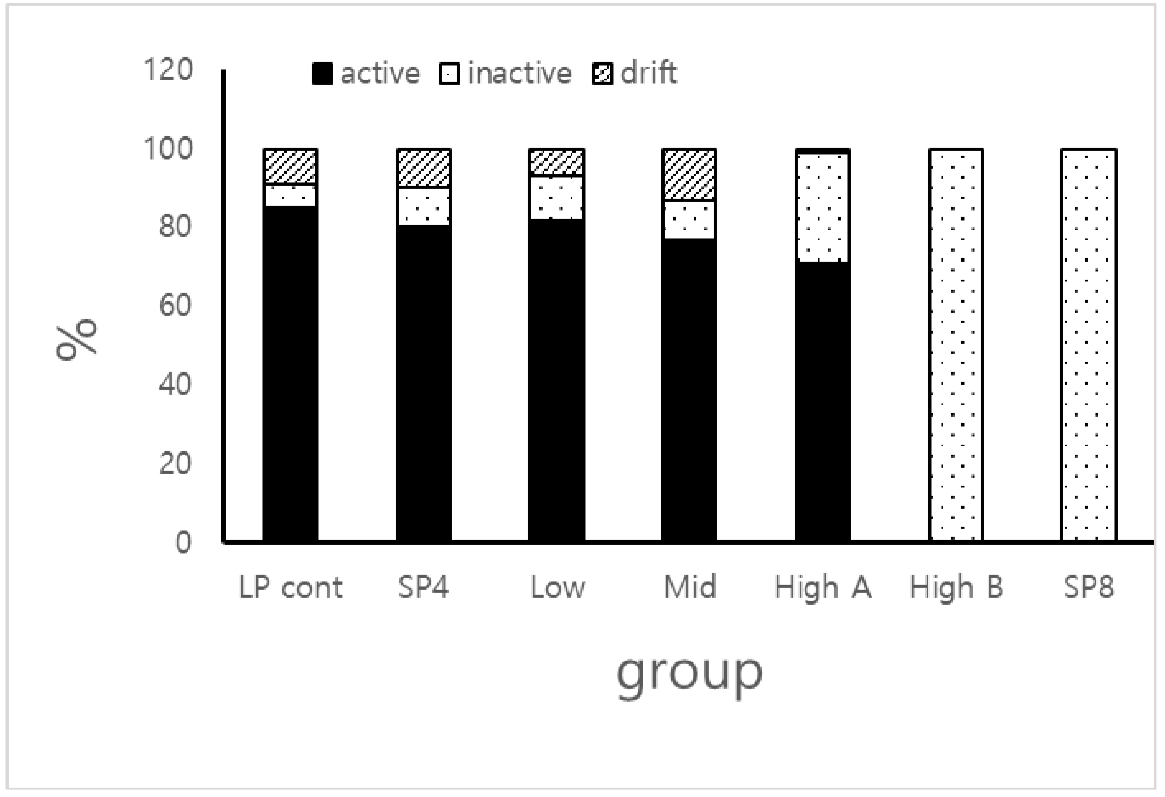
The various aspects of the seminiferous tubules were simply assorted into three categories in the rational analysis of the histologocal views, which were active (full spermatogenesis), inactive (completely arrested spermatogenesis), and drift (deviated germ cells from the original places). The categories were counted in the histologocal views and expressed as percentage (Fig. 9). More than 71% of the active seminiferous tubules were observed in LP control, SP4, low, middle, and high A groups. The tubules with active spermatogenesis were not found in small testicles of the high B and in SP8 groups. The various degree of inactive tubules (less than 10% in LP control, low, middle, and SP4 groups, and 28% in high B group) were counted. The 100% of inactive tubules were found in high B and SP8 groups. The drift, where some germ cells were absconded from the typical positions of seminiferous tubules, were observed in less than 13% in LP control, low, middle, high A, and SP4 groups. None of the drifts were witnessed in high B and SP8 groups. No spermatozoa were observed in high B and the SP8 groups.
DISCUSSION
In the present study that golden hamsters fed diet containing acetaminophen, spermatogenesis was appeared to be influenced. Some of the animals in high group showed entirely regressed testicles with tiny testicles. The outcome is compatible to testicles of animals housed in SP for 8 weeks where the spermatogenesis was distinctly arrested. The results implies that acetaminophen treated with diet could induce an antigonadotropic effect on the reproductive function in the hamsters.
The reproductive activity of golden hamsters has been well documented to be regulated by the photoperiod, as established by many previous investigations (Gaston & Menaker, 1967; Elliott, 1976; Reiter, 1980; Stetson & Watson-Whitmyre, 1984, 1986; Choi, 2013). In summer season, designated as LP, their reproductive functions are active with large testicles. Whereas in winter climate, designated as SP, their sexual activities are completely arrested with tiny testicles. The changes of testicular masses are distinctly distinguished in naked eyes in the bioassay of the animals. That was reason that the golden hamsters was used in this study. Moreover the seasonal phenomena are immitated by contolling timing schedules of lights in the animal facility. Thus both sexul active and inactive animal are simultaneously to be furnished.
Acetaminophen has a low solubility in various solvents. In order for hamster to give adequate dose of acetaminophen, the diet was reconstructed to contain acetaminophen to induce an effect. The diets were supplied and the amounts that they ate were daily checked in the morning. All animals in each groups ate nearly same amounts of diet except for the high group. The animals in the high group had significantly low amount of diet from the 3rd week and on. The reason that the animals took comparably low amount of diet might be the fragrance that the reconstructed diet gave off. The diet containing high omount of acetaminophen was slightly darker colour in naked eyes compared to the diet containing the middle and low amount of acetaminophen. The fragrance was not differently felt by human’s nose among the diets absorbing different concentrations of acetaminophen. In the same way the average body weights in all groups were increased except for the high group. The average body weight in high group was significantly (p<0.05) reduced. This consequence was consistent with the amount of food the animals ingested. Although the body weights were lessened in the animals in high group, they did not show any bezaare behavior in all time.
In the bioassay of non-reproductive organs, there were significant (p<0.05) reductions in the weights of liver and kidneys in high group in comparison to all other groups. The discrepance was relatively severe in the livers than the kidneys. These results were previously well substantiated in many reports (Davidson & Eastham, 1966; Boyer & Rouff, 1971; Mazer & Perrone, 2008; Lancaster et al., 2015; Du et al., 2016). The toxic effects of acetaminophen has been well documented in other reports (Boyer & Rouff, 1971; Mazer & Perrone, 2008; Olaleye & Rocha João, 2008; Lancaster et al., 2015; Rahimi et al., 2022; Chilvery et al., 2023). Nevertheless the damaging impacts of acetaminophen have been alleviated by various treatment (Aksu et al., 2016; Khayyat, 2021; Rahimi et al., 2022; Chilvery et al., 2023).
In the present investigation focused on the influence of acetaminophen on the reproductive activities, the weights of reproductive organs, including testicles, epididymis, and seminal vesicles were significantly lessened in high group. When the organs were examined individually, two entirely diffent situations were observed. The large testicles and the tiny testicles were detected, which were reproductively active testicles and reproductively regressed testicles, respectively. The completely involuted testicles shown by the animals in high group was thought as the direct impact of acetaminophen. On the other hand, the animals with large testicles in high group was thought not be influenced by the acetaminophen. It was regared as the difference of the individuals. The animals were treated with diet containing acetaminophen for 4 weeks. As a seasonal breeding animal, to make the testicles of golden hamsters to completely regress at least 8 weeks are needed. In 4 weeks exposure to SP the testicles still produce germ cells enegetically, indicating full spermatogenesis. The tiny testicles in high group showed the same aspects of spermatogenesis as the entirely regressed testicles in the aminals housed in SP for 8 weeks. Thus it is reasonale to consider that the regressed testicles in high group indicate different response compared to actions exerted by the animals exposed to SP. The consequences of total regression was inspected as the pure effects of acetaminophen. The similar experimental results were reported where the fertile fecundity was examined in the mice that fed acetaminophen-containing food (Reel et al., 1992).
When the seminiferous tubules were classified into active, inactive, drift, as mentioned in the serults section, the 100 percentage of the inactive class was counted in both in animals with tiny testicles in high group and the animals housed in SP for 8 weeks. The outcome is well consisten with the previous reports (Sinha Hikim et al., 1988; Seco-Rovira et al., 2015; Choi, 2019; Jeon et al., 2020). The percentage of inactive class in animals with large testicle in high group was also comparatively elevated in comparison to the animals of other groups with large testicles. The animals in high group fed the diet for 4 weeks. Thus these results indicate that the regressing process was accelerated in the animals in high group compared to the animals exposed to SP where it needs at least 8 weeks to reach to the total gonadal regression
Thus these results indicate that acetaminophen invokes the antigonadal effects and accelerates the regressing process of the testicles in the animals compared to the animals exposed to SP. Further investigations are required to find how acetaminophen affects the reproductive endocrine system.

