INTRODUCTION
Quantum dots (QDs) are nanoscale semiconductor particles that possess unique optical and electronic properties, including narrow emission bandwidth, tunable band gaps, and high quantum yield, enabling a wide range of biological and biomedical applications (Resch-Genger et al., 2008). However, conventional metal-based QDs, such as cadmium telluride and cadmium selenide QDs, can release free metal ions and induce reactive oxygen species (ROS), which can lead to DNA damage and organ toxicity (Tsoi et al., 2013).
To address these concerns, carbon quantum dots (CQDs), comprising sp2-hybridized carbon cores with surface bearing diverse functional groups (including heteroatom-containing moieties such as oxygen, nitrogen, sulfur, or phosphorus), have emerged as a promising class of nanomaterials with low intrinsic toxicity (Xiao et al., 2016). The functionalities of CQDs, including enhanced photoluminescence, redox stability, and biological reactivity, have led to their in biomedical imaging, biosensing, and targeted drug delivery (Mansuriya & Altintas, 2020). Nevertheless, their small size and high surface reactivity may promote biomolecules and transit across biological barriers, prompting investigation into whether such properties could affect germ cells and early embryos (Zhang et al., 2019; Wang et al., 2022).
Previous studies have reported that exposure to certain CQDs can induce oxidative stress, apoptosis, or endoplasmic reticulum (ER) stress in various cellular systems, indicating that their biological safety warrants careful evaluation (Zhong et al., 2024). Although CQDs have been widely applied in live-cell imaging and intracellular labeling, their effects on reproductive cells have not been systematically characterized. Our previous study showed that nitrogen-doped carbon quantum dots (NCQDs), in which nitrogen is introduced into graphitic domains, can regulate cellular proliferation and differentiation in embryonic stem cell-based models (Song et al., 2024), suggesting that they may be a promising tool for investigating functional modulation in reproductive cells. In particular, how CQDs influence sperm motility, fertilization potential, and subsequent embryonic development has not been systematically examined. Considering that CQDs can modulate cellular redox status and stress responses in other cell types (Almansa-Ordonez et al., 2020), it is essential to assess their potential impact on sperm activation and early embryonic development at the cellular level.
Sperm motility and capacitation are critical for successful fertilization, and impaired sperm function is a major cause of male infertility. Enhancing sperm motility and fertilization efficiency has therefore become an important goal in assisted reproductive technologies (ART) (Chakraborty & Saha, 2022). Various biological and chemical approaches have been explored to improve sperm function, including the use of antioxidants and metabolic modulators that influence mitochondrial activity and oxidative balance (Aitken & Curry, 2011; Amorini et al., 2021). Although these strategies have shown partial success, they are often limited by transient effects and inconsistent reproducibility. In this context, nanomaterials such as CQDs have attracted attention for their ability to interact with cells and modulate physiological activity at the nanoscale (Salvi et al., 2024). Given these properties, CQDs have been proposed as bioactive nanomaterials that could potentially enhance reproductive processes beyond imaging applications.
Because cellular stress responses play critical roles in gamete quality and embryonic viability, we focused on ER stress and unfolded protein response (UPR) markers primarily in early embryos (Schröder & Kaufman, 2005). Prior reports that QDs can induce oxidative stress and perturb ER homeostasis in non-reproductive systems motivate probing these pathways in reproductive contexts (Wang & Tang, 2018; Zhang et al., 2023).
In this study, we investigated whether carbon-based QDs affect sperm motility and in vitro fertilization (IVF) outcomes. Specifically, we assessed whether NCQDs modulate sperm activation, fertilization rate, and embryonic development in vitro by comparing NCQD-treated samples with untreated controls.
These findings broaden our understanding of nanomaterial–reproduction interactions and provide a conceptual framework for the safe integration of carbon-based nanomaterials into reproductive biotechnology. By revealing both the beneficial and adverse effects of NCQD exposure, this study lays the groundwork for developing optimized nanomaterial strategies that enhance fertility outcomes without compromising developmental integrity.
MATERIALS AND METHODS
NCQDs were synthesized using citric acid and urea as carbon and nitrogen precursors, following our previously reported microwave-assisted method. Briefly, equimolar concentrations of citric acid and urea were dissolved in deionized water and subjected to microwave heating to induce carbonization and nitrogen doping. The reaction mixture was then purified by centrifugation and dialysis to remove unreacted precursors. The resulting NCQDs exhibited an average particle diameter of approximately 3–5 nm distribution, as characterized by transmission electron microscopy (TEM). Surface functional groups, including amine-, amide-, and hydroxyl-containing moieties, were confirmed by X-ray photoelectron spectra and Fourier transform infrared spectroscopy (FT-IR) analysis.
All animal studies were conducted using six-week-old male and female Institute of Cancer Research (ICR) mice obtained from KOATECH (Pyeongtaek, Korea). Mice were maintained under controlled environmental conditions with food and water provided ad libitum and housed under a 12-hour light/dark cycle. For the experiments, one male mouse was used per CASA analysis, and each IVF experiments was performed using one male and six female mice. In total, 75 female and 25 male mice were used across all replicates. Animal care complied with the Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee (IACUC) ( JBNU 2022-050).
6-week-old ICR male mice without any prior treatment were sacrificed by CO2 inhalation. The epididymides were excised, and spermatozoa were collected into 100-μL drops of human tubular fluid (HTF; Cat. No. MR-070-D, Millipore, Burlington, VT, USA). The control group was incubated in HTF medium alone, while the treatment groups were incubated in HTF medium containing NCQDs. Each drop of HTF was covered with mineral oil to prevent evaporation and to maintain pH equilibrium during incubation. Sperm motility and kinematic parameters were assessed at designated time points (0.5, 1, 3, 6, 24, 30 h) using a SCA® computer-assisted sperm analysis (CASA) system. Rapid PR values were calculated on sperm showing a average path velocity (VAP)≥25 μm/s and straightness (STR)≥80%, as defined by CASA criteria. Hyperactivation and mucus penetration was evaluated according to the manufacturer’s default analytical settings based on the kinematic parameters in the SCA® CASA software (version 6.7, Microptic, Barcelona, Spain) (Mortimer et al., 2015).
For each measurement, sperm suspensions were loaded into a 20-μm depth 8-chamber slide (SC-20-01-08-B, IMV Technologies, L’Aigle, France) and analyzed 5 times per time point. All experiments were independently performed 5 times (n=5) under standard incubation conditions (37°C, 5% CO2).
6-week-old ICR female mice were intraperitoneally injected with 7.5 IU of pregnant mare’s serum (PMSG; Daesung, Korea) to stimulate follicular development. After 47 h, 7.5 IU of human chorionic Gonadotropin (hCG; CG5-1VL, Sigma-Aldrich, St. Louis, MO, USA) were intraperitoneally injected to induce superovulation.
On the following morning (16 h post- hCG), IVF dishes were prepared by placing 100-μL and 200-μL of HTF for sperm and oocytes, respectively. Each drop was covered with mineral oil and place them in an incubator. Sperm were collected as described above and incubated for capacitation at 37°C, 5% CO2 for 1 h. For oocyte collection, hormone-treated 6-week-old ICR female mice were sacrificed, and the ampullae of the oviducts were isolated. Cumulus-oocyte complexes were obtained by puncturing the ampullae with a needle and transferred into fresh HTF drops under mineral oil. After 1 h sperm capacitation, sperm was adjusted to oocyte and fertilization was allowed to proceed for 3 h 30 min. Two sperm concentrations (1×106 sperm/mL and 1×105 sperm/mL) were used in this study based on previous reports recommending 102–103 sperm/μL for optimal IVF outcomes (Hasegawa et al., 2014), as well as our preliminary optimization experiments. In our pilot tests, 1×106 sperm/mL consistently yielded >50% progression to the morula stage, whereas 1×105 sperm/mL resulted in <50% progression under our culture conditions. Accordingly, these two concentrations were selected to evaluate NCQD effects under both high- and low-efficiency fertilization conditions. Fertilized oocytes exhibiting pronuclei were washed in fresh HTF drops and subsequently cultured overnight in EmbryoMax® KSOM Mouse Embryo Media (MR-121, Millipore). These pronuclear-stage embryos were classified as the 1-cell stage. Embryonic development was monitored daily, and embryos were classified according to days post coitum (dpc). The day of fertilization (zygote) was designated as 0.5 dpc. At 1 dpc, embryos reached the 2-cell stage; at 2 dpc, the 4-cell stage; at 3 dpc, the morula stage; and at 4 dpc, the blastocyst stage.
Embryos at the late morula to early blastocyst transition stage (3.25 dpc) were collected and immediately placed into TRIzol reagent (FATRR 001, FAVORGEN, Ping Tung Agricultural Biotechnology Park, Taiwan) for RNA isolation. Total RNA was isolated using TRI-Reagent following the manufacturer’s instructions, after which reverse transcription was performed using a TOPscriptTM cDNA Synthesis kit (ENZ-KIT106-0200, ENZO, Farmingdale, NY, USA) according to the manufacturer’s manual. Quantitative polymerase chain reaction (PCR) was performed using a TOP real™ qPCR 2X PreMIX (RT500M, Enzynomics, Daejeon, Korea) and Rotor-Gene Q 2plex PCR machine (9001862, Qiagen, Hilden, Germany). Each cDNA (100 ng/μL) was subjected to RT-qPCR template. Real Time PCR was performed using a three-step cycling protocol (95°C for 15 min; 40 cycles of 95°C for 20 s, annealing at gene-specific temperatures for 30 s, and 72°C for 30 s), followed by a melt-curve analysis. The primer sequences used in this study are listed in Table 1. mGapdh was used as internal control to normalize the expression levels. The data were quantified using the 2-ΔΔCt method.
RT-qPCR, reverse transcription-quantitative polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Oct4, octamer-binding transcription factor 4; Cdx2, caudal-related homeobox transcription factor 2; Tead2, TEA domain transcription factor 2; Atf6, activating transcription factor 6; Chop, C/EBP homologous protein.
Embryos at the late morula to early blastocyst transition stage (3.25 dpc) were fixed in 10% formalin for 10 min and washed with PBS. After fixation, embryos were permeabilized with 0.25% Triton X-100 for 1 h and subsequently washed with PBS. Blocking was performed by adding blocking solution (5% BSA in PBS containing 0.3% Triton X-100, Triton, Parade, Mumbai) for 1 h at room temperature. Primary antibodies against OCT3/4 (1:2,000, sc-5279, Santa Cruz Biotechnology, Dallas, TX, USA) and CDX2 (1:10,000 dilution, EPR2764Y, Abcam, Cambridge, MA, USA) were diluted in antibody dilution buffer (1% BSA in PBS containing with 0.3% Triton X-100) and applied to the embryos overnight at 4°C. After washing with PBS containing 0.05% Tween-20 (PBS/T), samples were incubated with secondary antibodies (anti-mouse IgG-Fc, 1:200, A90-131D2, Bethyl; anti-rabbit IgG-Fc, 1:200, A120-111D3, Bethyl Laboratoies, Montgomery, TX, USA) for 1 h in the dark. Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) (1:50,000) for 10 min, followed by final washes in PBS/T. Immunostained embryos were observed under a fluorescence microscope. All washing steps were performed at room temperature for 5 min each.
The experimental data were presented on graphs as means±SEM. For CASA parameters, comparisons between control and treatment groups at each time point were analyzed using the paired t-test. IVF and developmental outcomes were evaluated using Fisher’s exact test to assess the developmental ability and subsequent IVF success rates of the embryos. For multiple group comparisons, one-way analysis of variance (ANOVA, GraphPad Software, San Diego, CA, USA) was conducted where applicable. Statistical significance was defined as * p<0.05; ** p<0.01; *** p<0.001.
RESULTS
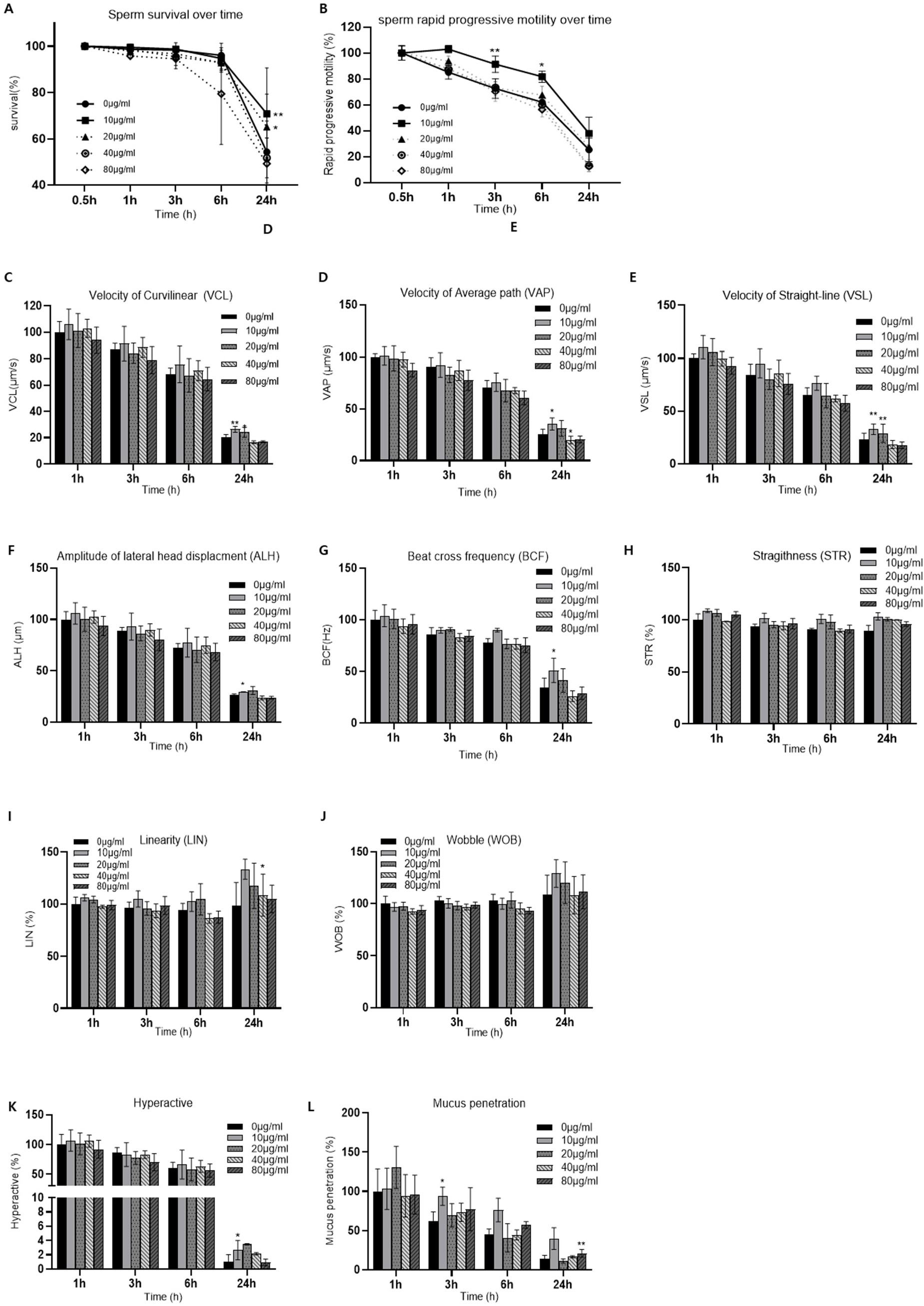
CASA was performed to evaluate the effects of NCQD exposure on sperm. Various concentration of NCQDs (10–80 μg/mL) were treated to sperm and motility parameters (survival rate, rapid progressive motility) and kinematic parameters [curvilinear velocity (VCL), VAP, straight-line velocity (VSL), amplitude of lateral head displacement (ALH), STR, linearity (LIN), beat cross frequency (BCF), wobble (WOB)] were measured (Supplementary Fig. S1). The survival rate was calculated as the proportion of motile spermatozoa with forward movement relative to the counted population as determined by CASA. Among the tested concentrations, 10 μg/mL of NCQD group exhibited most stable motility prolife, showing maintained relatively higher survival and motility over time compared with both control and the higher-dose groups. Therefore, 10 μg/mL of NCQDs were selected for subsequent analysis, which was conducted independently.
Sperm exposed to 10 μg/mL NCQDs maintained consistently higher survival rates than the control group throughout the incubation period, with a statistically significant difference at 6 h (p<0.05; Fig. 1A). The proportion of rapid progressive (rapid PR) motility remained higher in the NCQD-treated group compared with the control, and this enhancement reached statistically significant from 1 h to 30 h of incubation (p<0.05–0.01; Fig. 1B).
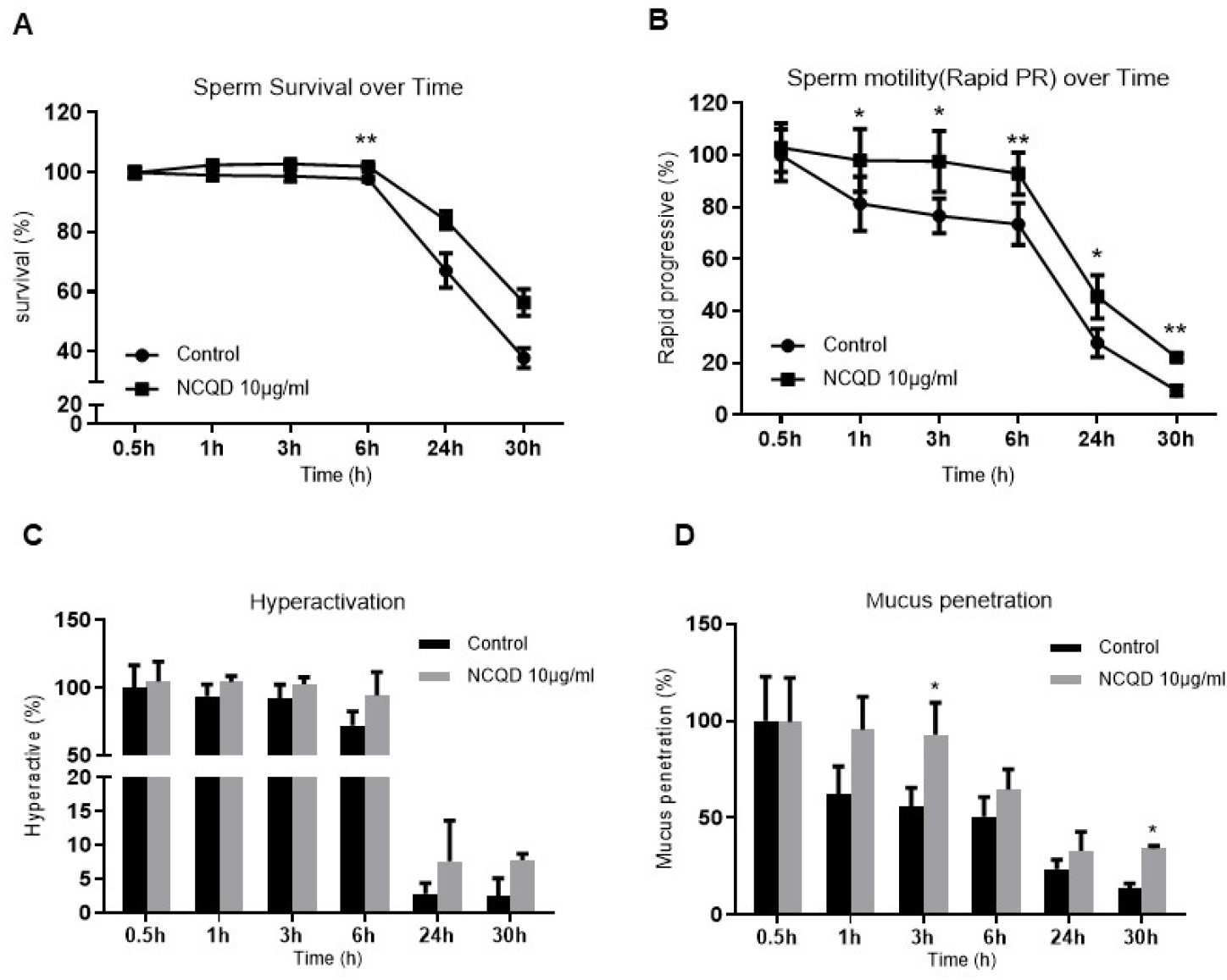
Functional indices reflecting sperm fertilizing potential, including hyperactivation and mucus penetration, were evaluated based on the motility and kinematic parameters. Hyperactivation exhibited a moderate increase in the NCQD-treated group compare with the control (Fig. 1C), and mucus penetration showed a marked increase throughout the incubation period, with statistically significant differences observed at 3 h and 30 h (p<0.05; Fig. 1D). Detailed quantitative data for all parameters are presented in Supplementary Table S1. These quantitative findings are supported by representative bright-field videos showing preserved sperm motility and survival in the NCQD-treated group after 30 h of incubation (Supplementary Fig. S2).
Collectively, these results indicate that low-dose NCQD exposure (10 μg/mL) is associated with improved sperm survival and supports motility performance over time, suggesting a stimulatory or protective effect on sperm function and capacitation.
To assess whether NCQD-mediated enhancement in sperm motility translates into improved fertilization and embryo development, IVF was performed using 1×106 sperm/mL in the presence of 10 μg/mL NCQDs. After fertilization, embryos were monitored through sequential developmental stages from zygote to blastocyst (Fig. 2A). Although 0.5 dpc represents zygotes at the pronuclear stage, the transition from 1-cell to 2-cell stage may occur spontaneously following minimal stimulation. Accordingly, to avoid misreading of early cleavage events, fertilization efficiency was evaluated based on the progression from the 2-cell to 4cell stage. The fertilization rate, expressed as the proportion of oocytes reaching 2-cell to 4-cell, was slightly higher in the NCQD-treated group than in the control. Developmental progression from 4-cell to morula was significantly improved, with 74.10% of embryos progressing in the NCQD groups compared with 53.06% in the control (p<0.01). In contrast, the transition from morula to blastocyst was markedly reduced in the NCQD-treated group (57.83%) compared with the control (82.69%, <0.01) (Fig. 2B).
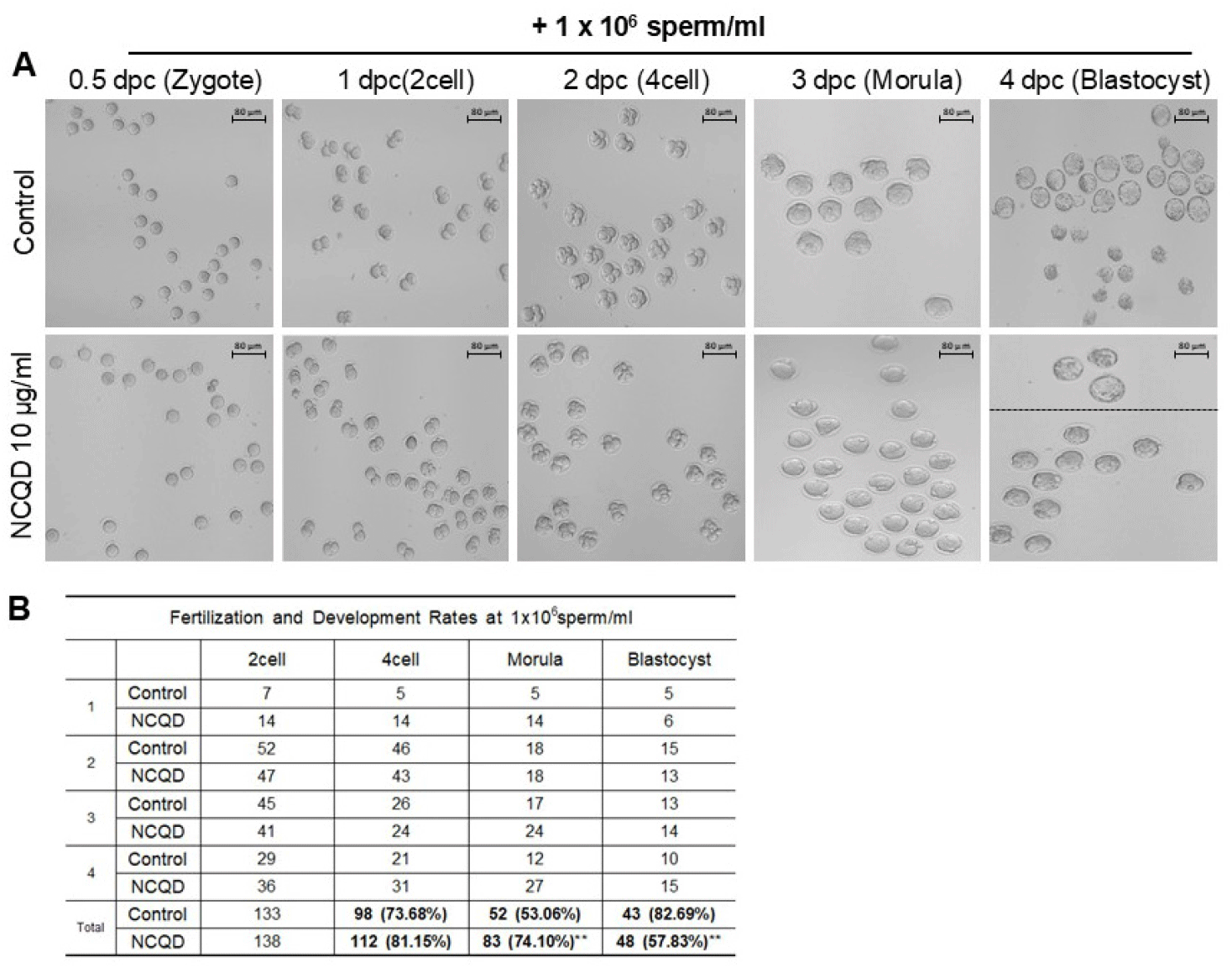
Collectively, these data indicate that low-dose NCQD treatment (10 μg/mL) is associated with improved fertilization and early cleavage progression, particularly enhancing compaction from the 4-cell to morula stage.
To evaluate whether NCQD-induced sperm activation could enhance fertilization and development outcomes under low-sperm, IVF was performed with 1×105 sperm/mL in the presence or absence of 10 μg/mL NCQDs (Fig. 3). At the 4 h after insemination, oocytes in the control group still retained partially intact cumulus cell layers, whereas those in the NCQD-treated group exhibited more rapid and efficient cumulus cell dispersal, and pronuclear-stage zygotes were observed (Fig. 3A). As a results, the fertilization rate was comparable between the control (79.37%) and NCQD-treated group (80.64%). However, a significant improvement was observed in the transition from the 4-cell to morula stage with 56.57% of embryos progressing in the NCQD-treated group compared with 44.63% in the control (p<0.05). In contrast, the proportion of embryos reaching the blastocyst stage slightly decreased in both groups (40.5% vs. 30.3% for control; Fig. 3B).
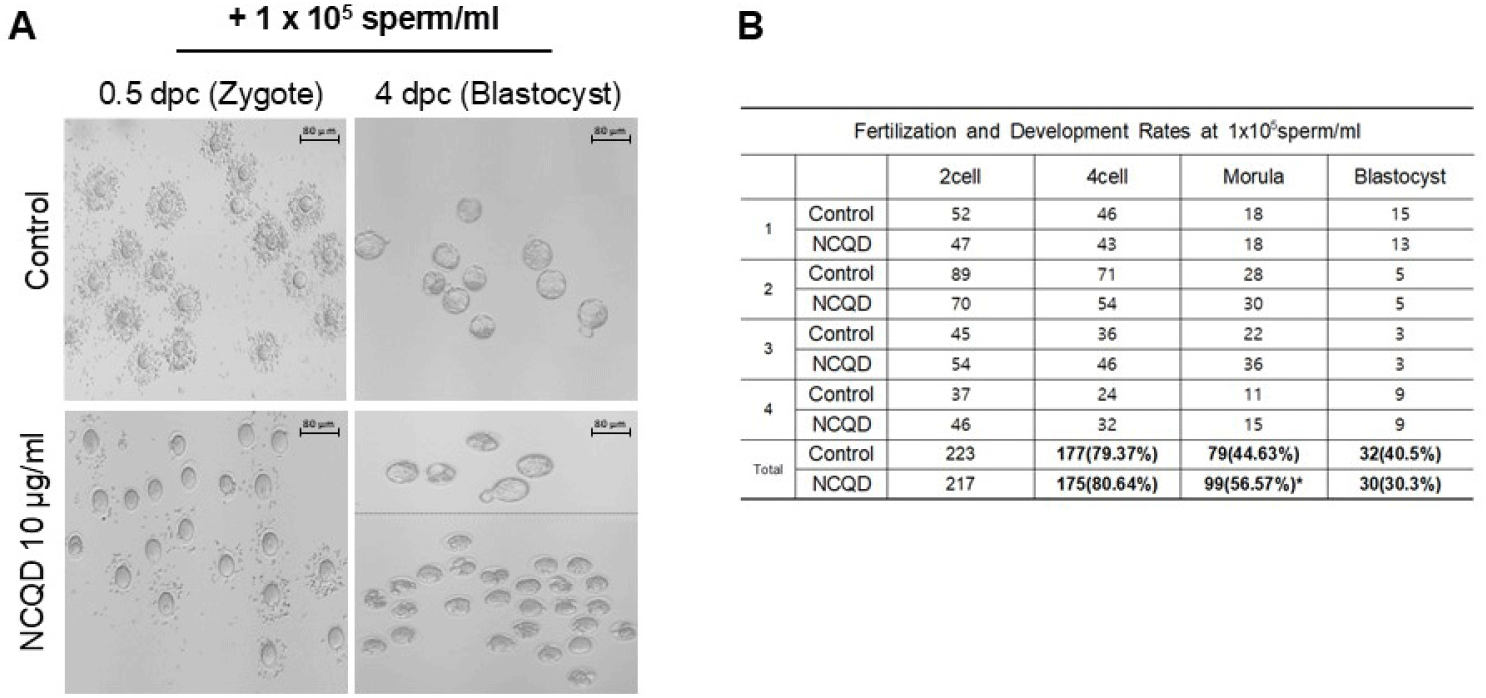
Collectively, these results indicate that, even under low-sperm IVF conditions, exposure to NCQDs is associated with more efficient sperm-oocyte interaction and early cleavage progression, particularly compaction to the morula stage, while blastocyst formation remains limited.
Preimplantation development is associated with the expression of Oct4 and Cdx2 and with cell-fate segregation into inner cell mass (ICM) and trophectoderm (TE). Therefore, we examined whether exposure to NCQDs affects this lineage-specification during the morula-to-blastocyst transition. To investigate how NCQD exposure influences lineage specification during preimplantation development, we focused on the morula-to-blastocyst transition stage (3.25 dpc), when the first cell-fate segregation into ICM and TE occurs. At this stage, oct4 expression persists in ICM progenitors, while CDX2 expression emerges in the outer TE cells, defining the onset of lineage differentiation.
RT-qPCR analysis revealed that expression of the ICM marker Oct4 was slightly decreased in NCQD-treated embryos compared with controls (Fig. 4A), whereas the TE-associated markers Cdx2 and Tead2 were significantly downregulated in the NCQD-treated group (p<0.05–0.01) (Fig. 4B and C).
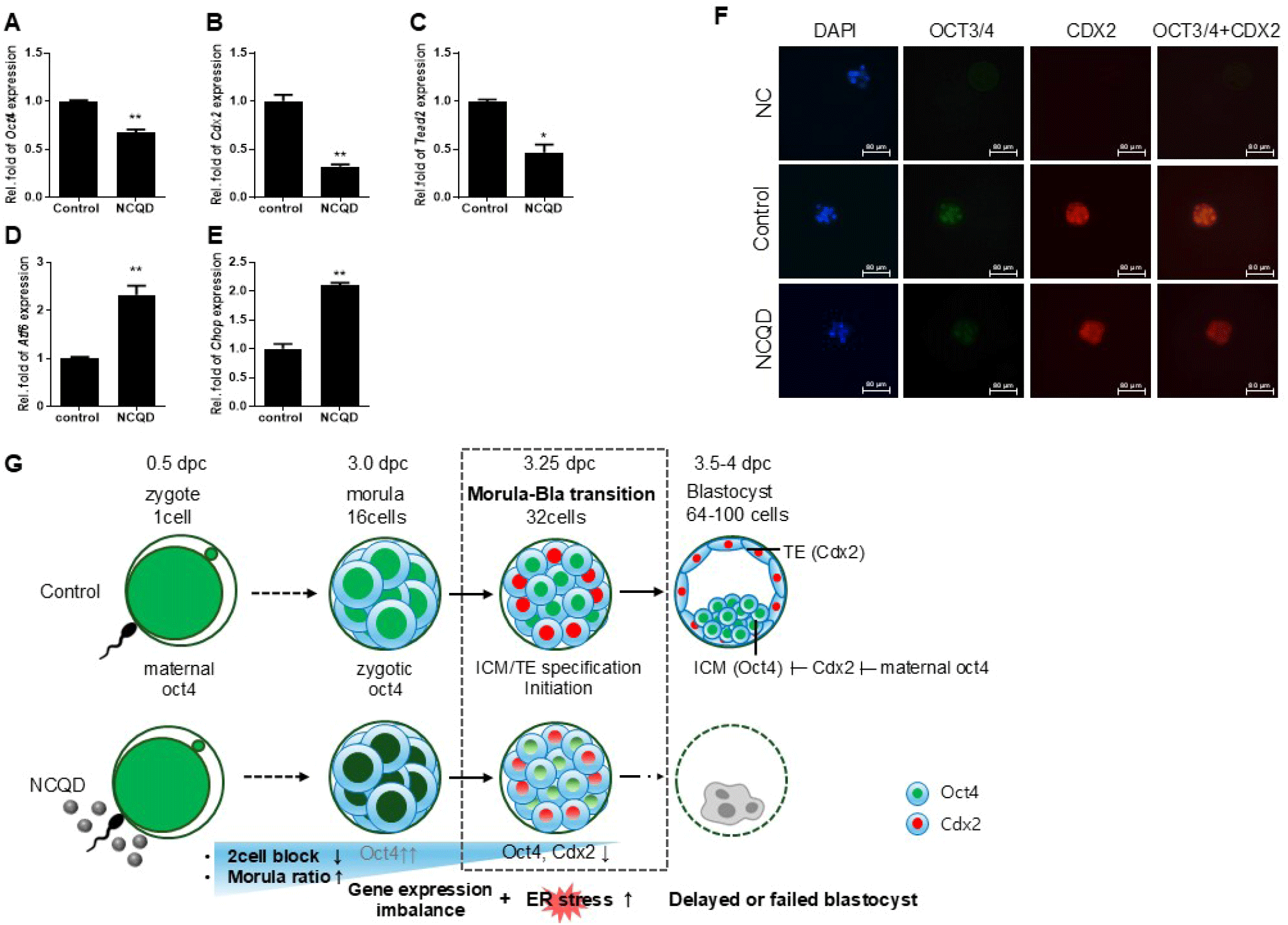
Consistently, immunocytochemistry showed decreased staining intensity of OCT3/4 and CDX2 protein in morula-to-blastocyst stage after NCQD treatment (Fig. 4F). The diminished CDX2 signal, particularly in the outer TE region, indicates impaired TE differentiation.
In addition, RT-qPCR results demonstrated upregulation of ER stress-related genes Atf6 and Chop in the NCQD-treated (p<0.05–0.01) (Fig. 4D and E), consistent with activation of ER stress pathway that may interfere with TE specification and blastocyst maturation.
Collectively, these results indicate that while NCQD exposure perturbs the balance between pluripotent ICM and differentiating TE lineages. NCQDs appear to support early embryo development, encompassing cleavage and compaction up to morula stage, as well as ICM maintenance. However, sustained exposure during the morula-to-blastocyst transition is associated with reduced TE gene expression, potentially through stress-mediated disruption of differentiation cues (Fig. 4G).
DISCUSSION
As CQDs have gained increasing attention to diverse biomedical and bio imaging applications, there is an increasing need to clarify their biological effects across different cellular environments. Although CQDs are generally considered less-toxic nanomaterials than metal-based quantum dots, their effects on reproductive systems remain incompletely understood.
In this study, NCQDs were synthesized from biocompatible, citric acid and urea, to evaluate their functional effects on reproductive cells, including sperm and embryos. Our results demonstrate that NCQDs exert stage-specific, biphasic, and concentration-dependent effects on reproductive cells, consistent with the dose-dependent responses previously reported in stem-cell systems (Song et al., 2024). These findings suggest that the mechanisms underlying CQD-induced cellular modulation in stem cells may also operate in germline and early embryonic contexts.
To objectively quantify these improvements, we used the CASA system, which provides precise and reproducible measurements of sperm kinematics and is widely used to assess fertilization potential (Yeste et al., 2018). At a low concentration (10 μg/mL), NCQDs significantly enhanced sperm motility, survival, and capacitation potential (Fig. 1), leading to improved fertilization and embryo development. It is well established that embryos generated under low-sperm IVF conditions often exhibit reduced developmental stability, as insufficient sperm numbers limit timely zona penetration, synchronized pronuclear formation, and coordinated early cleavage (Huang et al., 2015). This biological constraint explains the overall reduction in the 4-cell-to-morula transition observed in our low-sperm experiments (1×105 sperm/mL), even though NCQD treatment still improved the relative progression rate compared with the corresponding control group. Under reduced sperm concentration, NCQDs also accelerated cumulus cell degradation and promoted a rapid sperm acrosome reaction around the oocytes, indicating that enhanced sperm activation contributed to more efficient fertilization. Enhanced motility is generally indicative of improved cellular energy dynamics, driven by efficient mitochondrial function, which is essential for optimal sperm performance (Giaccagli et al., 2021). These improvements in sperm performance may arise from increased mitochondrial efficiency and redox homeostasis, as NCQDs can facilitate electron transfer and act as mild ROS scavengers at low levels (Li et al., 2023). Such modulation likely supports sustained ATP production and plasma membrane stability, thereby maintaining motility during prolonged incubation. Thus, the enhanced motility, capacitation, and survival observed in NCQD-treated sperm support the potential utility of NCQDs as bioactive agents for improving male fertility. Importantly, these beneficial effects also extend to early embryonic development, indicating broader reproductive relevance beyond gamete-level effects.
In the IVF model, NCQD treatment significantly increased fertilization rates (Fig. 2). Statistical comparison between the control and NCQD-treated groups was performed using the Fisher’s exact test, a method commonly applied to evaluate success or failure during developmental stage transition (Li et al., 2021; Tomic et al., 2025). In mouse embryos, the 2-cell block represents a critical developmental bottleneck, and successful progression beyond this stage is essential for normal preimplantation development (Matsukawa et al., 2002). In our study, NCQD treatment effectively facilitated the 2-cell-to-4-cell transition, resulting in a higher proportion of embryos advancing to the morula stage. These findings indicate that NCQDs not only improve gamete quality but also promote early cleavage-stage development.
However, despite this early-stage improvement, a noticeable delay was observed in the transition to the blastocyst stage. Proper blastocyst formation requires precise coordination between the ICM and the TE (Wang et al., 2010; Wigger et al., 2017). Oct4 is expressed from the oocyte stage onward and plays a central role in maintaining pluripotency within the ICM (Wu & Schöler, 2014) and zygotic Oct4 expression is activated from the 8-cell stage and maintained in all blastomeres throughout the morula. Oct4 is considered a master transcriptional regulator of pluripotency (Han et al., 2022). In contrast, the TE lineage is specified by Cdx2, which acts in a reciprocal, mutually repressive interaction with Oct4 to ensure accurate lineage segregation (Niwa et al., 2005). Cdx2 is essential for the segregation of the ICM and TE lineages (Strumpf et al., 2005). Specifically, Cdx2 promotes the expression of TE-specific genes and represses Oct4 transcription by preventing its autoregulatory activation (Huang et al., 2017).
Our previous study demonstrated that NCQDs maintain elevated Oct4 mRNA expression under mild differentiation conditions and enhance pluripotency in mouse embryonic stem cells. Considering this, it is plausible that NCQD exposure in early embryos initially induces disproportionate or prolonged Oct4 activity, which could disturb the transcriptional cues required for subsequent lineage transition. Such an imbalance may interfere with the activation of TE-specific genes and ultimately disrupt coordination between ICM and TE differentiation programs. In the present study, both Oct4 and Cdx2 expression were reduced at the morula-to-blastocyst transition (Fig. 4B and C), suggesting a global downregulation of lineage-specifying factors. This pattern implies that earlier pluripotency imbalance at the early morula stage may impair subsequent lineage commitment essential for proper blastocyst development.
Moreover, ER stress marker expression was elevated in embryos transitioning from the morula to blastocyst stage following NCQD treatment, supporting previous findings that link ER stress with embryonic developmental arrest (Lin et al., 2019; Capatina et al., 2021). This stage-specific disturbance underscores the biphasic or dual-phase nature of NCQD exposure: it enhances early proliferation but may impair later differentiation by inducing cellular stress. Embryos are inherently more sensitive than other reproductive cells because of their rapid metabolic and transcriptional transitions during development. Such heightened sensitivity likely amplifies NCQD-induced stress, leading to distinct stage-specific outcomes. Therefore, precise optimization of NCQD concentration and exposure timing during IVF procedures is necessary to ensure proper lineage specification.
Previous studies have largely focused on the cytotoxicity and fluorescence properties of NCQDs rather than their reproductive effects. While most reports describe NCQDs as biocompatible at low doses, only a few have examined their potential bioactivity in gametes or embryos. In our previous work, we investigated the effects of NCQDs on proliferation and differentiation in embryonic and adult stem cells. In contrast, the present study establishes a direct link between NCQD-induced sperm activation and subsequent embryonic development, thereby providing a functional continuum between gamete quality and developmental competence.
This study was conducted under in vitro conditions and focused primarily on early developmental stages. Future investigation would extend to in vivo models to evaluate reproductive toxicity, implantation success, and offspring outcomes. Mechanistically, transcriptomic and proteomic profiling of embryos exposed to NCQDs will be essential to delineate the molecular networks underlying ER stress and lineage specification.
These results provide novel insights into how carbon-based nanomaterials interact with gametes and preimplantation embryos. The ability of NCQDs to enhance sperm activity and early embryo development suggests potential applications in ART, such as improving fertilization efficiency or maintaining sperm viability during storage and handling. However, the stage-specific developmental inhibition observed at later stages emphasizes the need for careful optimization of dosage and exposure timing. From a broader perspective, the data also highlight the importance of evaluating nanomaterial bioactivity not only in stem cells but also within reproductive system, where subtle molecular perturbations can have long-term developmental consequences.
SUPPLEMENTARY MATERIALS
Supplementary materials are only available online from: https://doi.org/10.12717/DR.2025.29.4.137.