INTRODUCTION
Nucleobindin precursor protein, which was identified for the first time in the human and mouse cell lines, is known to have two isotypes, nucleobindin 1 (NUCB1) and nucleobindin 2 (NUCB2) (Miura et al., 1992; Kanai & Tanuma, 1992; Barnikol-Watanabe et al., 1994). However, NUCB2 only functions physiologically in humans and rodents (Miura et al., 1992). NUCB2 produces nesfatin-1 (residues 1-82), nesfatin-2 (residues 85-163), and nesfatin-3 (residues 166-396) by the enzyme pro-hormone convertase (PC)-1/3 after putative post-translational processing. Until now, a physiological activity has only been demonstrated for nesfatin-1 (Oh-I et al., 2006; Stengel et al., 2012).
Nesfatin-1 is initially known to be expressed in the hypothalamic nuclei such as arcuate nucleus, paraventricular nucleus, supraoptic nucleus, lateral hypothalamic area and zona incerta in rats (Brailoiu et al., 2007; Chen et al., 2012; Foo et al., 2008; Fort et al., 2008; Goebel et al., 2009; Goebel et al., 2011; Kohno et al., 2008; Stengel et al., 2010; Xu et al., 2009). Recent studies showed that a large amount of the nesfatin-1 expression was detected in the digestive system as well as in the brain. It has also been reported that the nesfatin-1 generated in the gastric organs, by acting in the brain, reduces food intake and suppresses the motilities of the stomach-duodenum (Prinz et al., 2016; Jiang et al., 2016). Nesfatin-1 immunoreactive cells are also co-localized with insulin in pancreatic beta-cells of mice and rats, suggesting a potential role for nesfatin-1 in pancreatic islet and glucose homeostasis (Shimizu & Osaki, 2014; Gonzalez et al., 2009). Furthermore, nesfatin-1 was detected in the adipose tissues of humans and rodents, showing that it was expressed more in the subcutaneous than in the visceral fat (Shimizu et al., 2016).
Recent studies have demonstrated that nesfatin-1/ NUCB2 are expressed in the reproductive system (Garcia-Galiano et al., 2010). We have also been shown that nesfatin-1/ NUCB2 mRNA and protein are expressed in the mouse hypothalamus and pituitary and its expression levels were regulated by the gonadotropin (Kim et al., 2014; Chung et al., 2015). Moreover, we showed that the expression of nesfatin-1/NUCB2 in the pituitary and hypothalamus is regulated by estradiol and progesterone secreted by the ovary (Chung et al., 2015). Therefore, we investigated whether the expression of nesfatin-1/NUCB2 in the pituitary and hypothalamus is affected by the testosterone produced by the testis in the male mouse.
MATERIALS AND METHODS
Eight-week-old male ICR mice were purchased from KOATECH (South Korea) and housed in groups of five per cage under controlled illumination (12:12 h light/dark cycle, lights on/off: 6 h/18 h) and temperature (22±2℃). Animals were fed a standard rodent diet and tap water ad libitum. Animal care and experimental procedures were approved by the Institutional Animal care and the use committee at the Seoul Women’s University in accordance with guidelines established by the Korea Food and Drug Administration.
Mice were castrated and rested for 48 h followed by intraperitoneal injection (100 μL/mouse) subcutaneously with sesame oil alone and with testosterone (300 ng/100 μL; Sigma, St. Louis, MO) dissolved in sesame oil. Mice were euthanized by CO2 anesthesia followed by cervical dislocation, and then the cerebrum, hypothalamus, pituitary gland, and stomach were collected 24 h after treatment. We obtained the fundus part of the stomach and the front part of cerebrum. For dissection of the hypothalamus and pituitary gland, we removed brain from skull and placed ventral side up. Using curved forceps, we took out the pituitary gland from hypophyseal fossa, and then pushed the curved part of forceps down around the hypothalamus and pinched it out.
The hypothalamus and pituitary glands were gently dissected away from the other tissues, put on a nylon mesh sheet in a 24-well culture plate (Becton Dickinson Labware, Franklin Lakes, NJ), and cultured in 200 μL/well serum-free DMEM/F12 medium (Invitrogen, Groningen, The Netherlands) containing antibiotics (Invitrogen). The hypothalamus and pituitary glands were treated with 10-–8, 10-–7, and 10-–6 M testosterone (Sigma, St. Louis, MO) for 24 h.
The cerebrum, hypothalamus, pituitary gland, and stomach were homogenized with RNA isoplus (TaKaRa Bio, Shiga, Japan). After chloroform extraction and isopropyl alcohol precipitation, RNA was dissolved in RNase-free DEPC (TaKaRa Bio, Shiga, Japan) solution. The RNA concentrations were measured with the Nano-drop (Thermo Fisher Scientific Inc., Waltham, MA). First strand cDNA synthesis was performed using the extracted RNA and oligo dT, followed by double-strand synthesis in RT buffer (Invitrogen, Carlsbad, CA) with dNTP (BIO BASIC Inc., Ontario, Canada) and RTase (Invitrogen, Carlsbad, CA).
Conventional PCR was performed in buffer solution containing template cDNA, Taq polymerase (BIONICS, Korea), dNTPs (BIO BASIC Inc., Ontario, Canada) and each primer. Primers were designed for NUCB2 and β-actin on the basis of the mouse cDNA sequences. The following primer pairs were used: NUCB2 forward 5-TTTGAA-CACCTGAACCACCA-3; reverse 5-TGGTCTTCGTGCTTCCTCTT-3 and β-actin forward 5-CTCTTTGATGTCACGCACGATTTC-3; reverse 5-ATCGTGGGCCGCTCTAGGCACC-3 primers (BIONICS, Korea). The optimum temperature cycling protocol was used as 95℃ for 15 s, 60℃ for 30 s and 72℃ for 30 min, using the Gene Pro thermal cycler (Bioer, China). The reaction products were run on a 2% agarose gel and visualized with ethidium bromide to check the length of the amplified cDNA.
qRT-PCR was performed in buffer solution containing template cDNA, SYBR Green (Roche, Manheim, Germany), and each primer. Primer pairs were as follows: NUCB2 forward 5-AAAACCTTGGCCTGTCTGAA-3; reverse 5-CATCGATAGGAACAGCTTCCA-3 and GAPDH forward 5-TTGATGGCAACAATCTCCAC-3; reverse 5-CGTCCCGTGACAAAA-TGGT-3 (BIONICS, Korea). The optimum temperature cycling protocol was determined to be 95℃ for 10 s, 60℃ for 10 s and 72℃ for 10 s using the Light Cycler 480 Real-time PCR System (Roche, Manheim, Germany).
Hypothalamus, pituitary, and stomach, and fat were quickly removed and extracted the protein with EDTA homogenization buffer, the samples were SDS-PAGE and transferred to the PVDF membrane. The membrane was treated in a blocking solution and incubated with rabbit anti-rat nesfatin-1 antibody (H-003-22, Phoenix Pharmaceuticals) / anti-mouse β-actin antibody (sc-47778, Santa Cruz Biotechnology) followed by incubation with donkey anti-rabbit IgG-HRP (sc-2313, Santa Cruz Biotechnology) / donkey anti-mouse IgG-HRP (sc-2096, Santa Cruz Biotechnology), respectively. By using the ECL Plus Western Blotting Detection Reagents (Amersham; GE Healthcare), the membrane was detected to investigate the expression level of nesfatin-1 protein.
The tissues of hypothalamus and pituitary gland were fixed in 4% paraformaldehyde buffer saline for 2 h. The tissues were rinsed in ethanol series to remove fixative residues, embedded in paraffin block. The tissues blocks were cut 10 μm sections using a microtome, deparaffinized, and rehydrated with graded xylene-alcohol series, and then washed with PBS before immunostaining. The sections were incubated with rabbit anti-rat nesfatin-1 polyclonal antibody (Phoenix Pharmaceuticals, INC., Burlingame, CA), followed by incubation with Alexa fluor 488 conjugated goat anti-rabbit IgG (Jackson immunoresearch laboratory, West grove, PA). The sections were counter stained with DAPI (4',6-diamidino-2-phenylindole; Sigma, St. Louis, MO) for 10 min and mounted on the slides with mounting medium (Vector laboratories, INC., Burlingame, CA), and then observed under fluorescence microscopy (Axioskop2, Carl Zeiss, Germany).
RESULTS
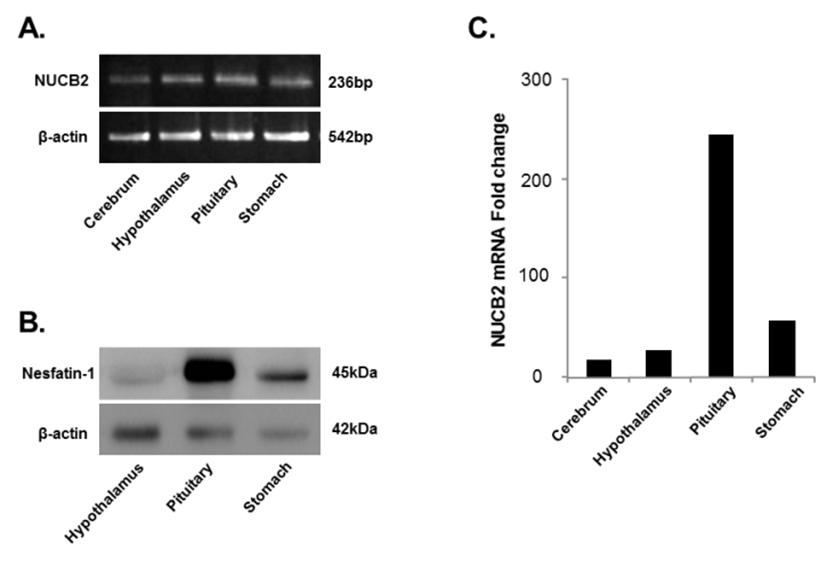
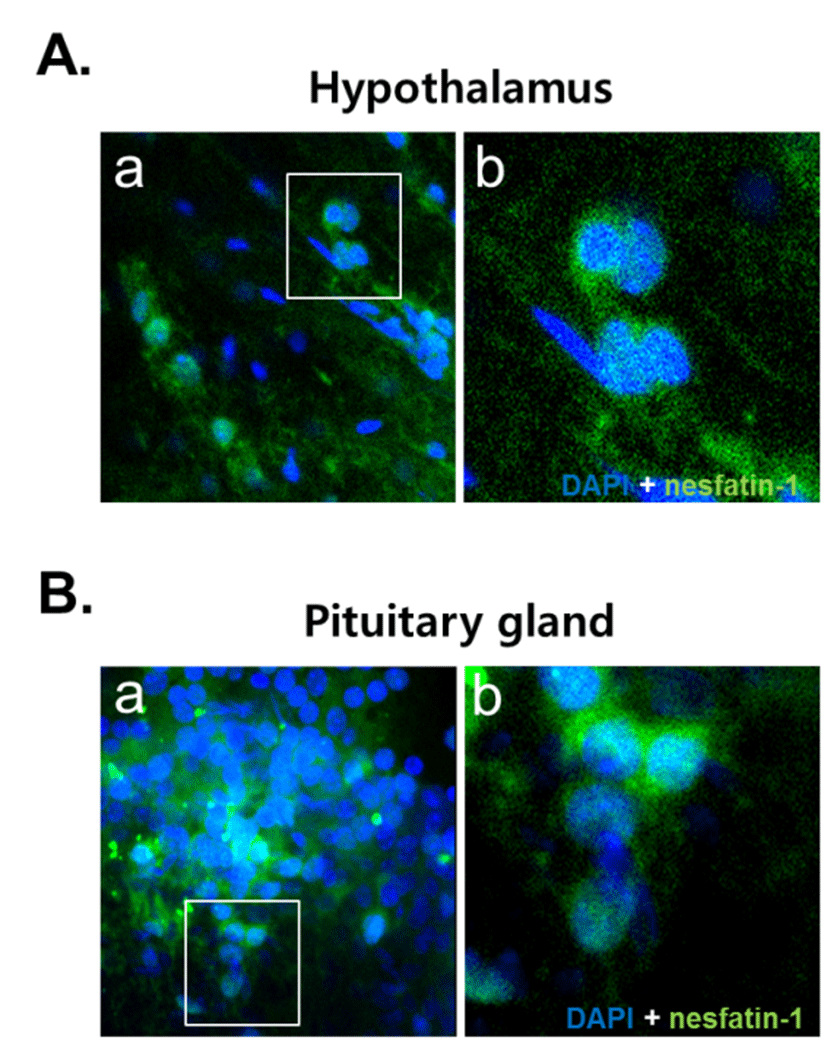
NUCB2 mRNA expression in the cerebrum, hypothalamus, pituitary, and stomach was detected by conventional PCR and qRT-PCR. We found that the NUCB2 gene was expressed in all tissues and its mRNA expression was significantly higher in the pituitary gland than in the cerebrum, hypothalamus, and stomach (Fig. 1A and C). Western blots confirmed that, similar to NUCB2 mRNA expression, nesfatin-1/NUCB2 protein was highly expressed in the pituitary gland (Fig. 1B). Immunohistochemical staining showed that nesfatin-1/NUCB2 protein was localized in many cells within the anterior pituitary gland (Fig. 2).
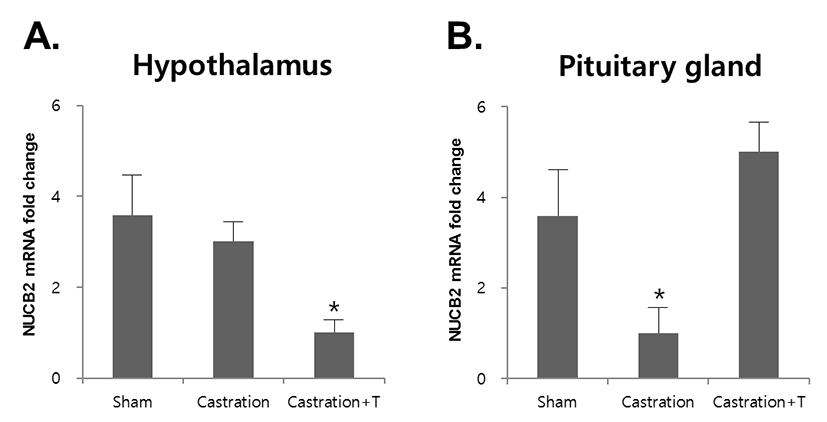
To assess whether NUCB2 mRNA expression in the hypothalamus and pituitary gland was regulated by testosterone secreted by the testis, mice were castrated and injected with testosterone. The expression of NUCB2 mRNA in the hypothalamus was not changed after castration, but its expression was significantly decreased after injection with testosterone (Fig. 3A). In the pituitary gland, the expression of NUCB2 mRNA was significantly decreased after castration and increased after injection with testosterone (Fig. 3B).
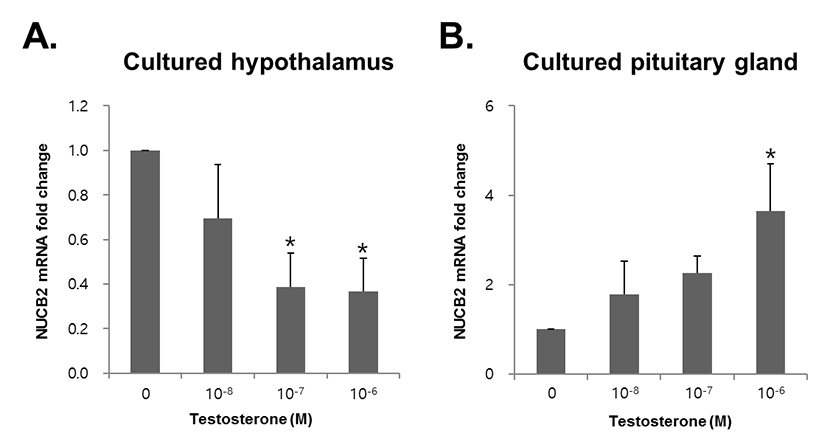
To investigate whether testosterone directly regulated expression of NUCB2 mRNA, we treated testosterone to mouse hypothalamus and pituitary gland tissues cultured in vitro. NUCB2 mRNA expression in the hypothalamus was significantly decreased with 10−6 and 10−7 M testosterone (Fig. 4A). By contrast, NUCB2 mRNA expression in the pituitary gland was increased in dose-dependent manner, showing significant increase in 10−6 M testosterone treatment (Fig. 4B).
DISCUSSION
Nesfatin-1 protein produced by neurons in the hypothalamus is known to be secreted into cerebrospinal fluid, and be controlling of appetite and energy metabolism (Oh-I et al., 2006). Recently, it has been reported that nesfatin-1/NUCB2 mRNA is expressed in gastric organs such as stomach and pancreas (Stengel et al., 2009), in adipose tissues of humans and rodents (Garcia-Galiano et al., 2010) and in the male and female mouse reproductive organs (Kim et al., 2010; Kim et al., 2011). Our previous study has demonstrated that nesfatin-1/NUCB2 expression level is higher in the pituitary gland compared to other organs and its expression is regulated by 17β-estradiol and progesterone secreted from the ovary (Chung et al., 2015). However, currently no data exist on the expression of nesfatin-1/NUCB2 and its regulation mechanism in the pituitary of male mouse. Therefore, in the present study we examined whether nesfatin-1/NUCB2 is present in the male mouse pituitary and if its expression is regulated by testosterone.
To examine the relative expression of nesfatin-1/NUCB2 mRNA and protein in the pituitary, we performed conventional PCR using total RNA extracted from the cerebrum, hypothalamus, pituitary, and stomach. The conventional PCR analysis showed that NUCB2 mRNA expression is detected in all tissues. The qRT-PCR and Western blotting data showed that the expression of nesfatin-1 protein and NUCB2 mRNA were higher in the pituitary gland than in other tissues. It is well known that nesfatin-1/ NUCB2 mRNA is abundantly expressed in the hypothalamus (Oh-I et al., 2006). Furthermore, recent reports have shown that nesfatin-1/NUCB2 is also expressed in the pituitary (Foo et al., 2008). These results raise the possibility that nesfatin-1 produced in the brain may control the metabolic homeostasis not only through the hypothalamus, but also through the pituitary. In other words, nesfatin-1 protein produced by the pituitary may move to target cells in the various organs through the bloodstream, similar to other hormones produced by the pituitary gland.
We also investigated whether testosterone regulates nesfatin-1/NUCB2 expression in hypothalamus and pituitary gland. In our in vivo experiment, NUCB2 mRNA expression was dramatically decreased in the pituitary gland after castration and significantly increased with testosterone, suggesting testosterone regulate NUCB2 mRNA expression in the pituitary gland. In the hypothalamus gland, NUCB2 mRNA expression was not changed after castration, but significantly decreased with testosterone, suggesting testosterone also regulate NUCB2 mRNA expression in the hypothalamus. Many cells in the hypothalamus and pituitary gland contain testosterone receptors (Jin and Yang, 2014), indicating that injected testosterone may affect the activity of both the hypothalamus and pituitary gland by binding to the receptor.
Next, we conducted an in vitro experiment to examine the direct effects of testosterone on NUCB2 mRNA expression in the cultured hypothalamus and pituitary gland. The expression of NUCB2 mRNA in the cultured hypothalamus tissues was significantly decreased with testosterone in dose-dependent manner. These results suggest that NUCB2 mRNA expression in the hypothalamus is directly down-regulated by testosterone. By contract, in the cultured pituitary gland tissues, NUCB2 mRNA was significantly increased with testosterone in dose-dependent manner. The present results show the possibility that the reduced nesfatin-1/NUCB2 expression in the hypothalamus in response to testosterone, which is produced and secreted by the Leydig cells in testis, may contribute to feeding behavior in male and even in female mice. Testosterone has been known to influence appetite control and feeding behavior (Iwasa et al., 2016; Bautista et al., 2012). Hypothalamic mRNA levels of ERα, which plays pivotal roles in regulation of body weight and metabolism, were decreased by chronic testosterone administration in rats (Iwasa et al., 2016). In female rats following ovariectomy, a stabilization of food intake was not observed, but energy intake was increased. By contrast, castration in males induced loss of body weight, whereas castrated males given testosterone showed complete restoration of eating activity (Ferreira et al., 2012). We also have previously reported that NUCB2 expression in the pituitary gland of female mice was dramatically decreased after ovariectomy and increased with injection of progesterone and estradiol, respectively. Furthermore, the in vitro experiment to elucidate the direct effect of progesterone and estradiol on NUCB2 mRNA expression showed NUCB2 mRNA expression was significantly increased with estradiol and decreased with progesterone alone and progesterone and estradiol in cultured pituitary tissue (Chung et al., 2015). The present study demonstrated that nesfatin-1/NUCB2 was highly expressed in the mouse pituitary and was regulated by testosterone. Recently, it is reported that nesfatin-1 production is greater in the reproductive organs, such as the seminal gland, testis, uterus, and ovary in rats with metabolic syndrome (Kim et al., 2010; Kim et al., 2011; Kim et al., 2014). These results suggest that metabolic syndrome could also influence the nesfatin-1/NUCB2 expression in the pituitary gland regulating appetite and energy homeostasis. Estradiol has been known to inhibit feeding in rats with a reduction of food intake around the time of ovulation, when estradiol presents its highest levels. Men with higher testosterone levels compared to women accumulate more fat in the intraabdominal depot, suggesting that testosterone stimulates feeding (Bautista et al., 2012).
The present study demonstrated that nesfatin-1/NUCB2 was expressed in the hypothalamus and pituitary gland of male mouse and its mRNA expression in the hypothalamus and pituitary gland was regulated by testosterone. This data suggests that reproductive-endocrine regulation through hypothalamus-pituitary-testis axis may contribute to nesfatin-1/NUCB2 expression in the hypothalamus and pituitary gland.

