INTRODUCTION
Nucleobindin-2 which is a calcium-binding protein produced by the expression of the NUCB2 gene is known to function physiologically only in humans and rodents (Miura et al., 1992; Barnikol-Watanabe et al., 1994). Nucleobindin-2 is converted nesfatin-1, nesfatin-2 and nesfatin-3 by the enzyme prohormone convertase-1/3 and only nesfatin-1 is known to have physiological activity (Oh-I et al., 2006; Gonzalez et al., 2010; Stengel et al., 2012). Nesfatin-1 is known to be first expressed in the hypothalamus, but has now been reported to be expressed in various organs, such as pancreas (Gonzalez et al., 2009), intestine (Zhang et al., 2010; Prinz et al., 2016; Jiang et al., 2016), and fat (Shimizu et al., 2016). Recent studies have shown that NUCB2/nesfatin-1 is expressed in the reproductive system of rodent and human (Garcia-Galiano et al., 2010; Catak et al., 2019). Our previous reports showed also that NUCB2/nesfatin-1 is expressed in the reproductive organs of mouse and is involved in the regulation of ovarian and uterine function along the hypothalamus-pituitary-ovary (Kim et al., 2014; Chung et al., 2015).
Oviduct is a passage through which the ovum released from the ovaries moves. The ovum enters the infundibulum and moves by cilia on the inner wall of the oviduct. The ovum meet sperms entered through the uterus and is fertilized in the ampulla of the oviduct. A fertilized zygote begins to divide into daughter cells about 30 hours, then develops into the blastocyst under the action of various substances secreted from the oviduct. Oviductal secretion affects embryo cleavage and development with various proteins and growth factors (Li & Winuthayanon, 2017). Oviduct-specific glycoprotein (OVGP1) is a high molecular weight chitinase-like protein belonging to GH18 family. It is secreted by non-ciliated epithelial cells of oviduct during estrous cycle providing an essential milieu for fertilization and embryo development (Choudhary et al., 2019). Glycodelin-A which is a type of glycoprotein produced from the oviduct inhibits fertilization and suppresses immune responses (Yeung et al., 2009). Recently, osteopontin (OPN) known to play significant roles in the bone remodeling is also detected in the oviduct and it plays an important role in ovum function and embryo development, as the expression is regulated by estrogen during estrous cycle and embryo in early pregnancy (Liu et al., 2015). On the other hand, ghrelin which is known as an appetite regulator has been reported to secrete from the oviduct and to regulate reproductive function (Deaver et al., 2013). Abnormal concentration of ghrelin in serum exerts negative effects on fertilization, implantation, and embryo/fetal development periods in mouse, supporting the hypothesis that ghrelin has a physiological role in early gestational events (Luque et al., 2014).
In the previous reports, we demonstrated that NUCB2/nesfatin-1 is expressed in the ovary and uterus of mouse reproductive system and its expression is regulated by gonadotropins and sex steroid hormones. Based on the fact, we hypothesized that NUCB2/nesfatin-1, one of the appetite-regulating hormones, can be expressed in the oviduct like ghrelin and its expression is regulated by sex steroid hormones. Therefore, we investigated whether NUCB2/nesfatin-1 is expressed in the oviduct by qRT-PCR, western blotting, and immunohistochemical staining. In addition, NUCB2/nesfatin-1 levels were measured in the oviducts of mice injected with PMSG and hCG or of ovariectomized mice injected with 17β-estradiol (E2) and progesterone (P4).
MATERIALS AND METHODS
Eight-week-old female ICR mice were purchased from KOATECH (Pyeongtaek, Korea) and housed in groups of five per cage under controlled illumination (12:12 h light/dark cycle, lights on/ off: 6 h/18 h) and temperature (22±2°C). Animals were fed a standard rodent diet and tap water ad libitum. Animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at the Seoul Women’s University in accordance with guidelines established by the Korea Food and Drug Administration.
PMSG (5 IU) and hCG (5 IU) were injected at an interval of 48 h. Pituitary, oviduct, ovary, uterus and muscle were collected 18 h after hCG injection and analyzed for mRNA and protein. Mice were anesthetized by intraperitoneal injection of a combination of ketamine and xylazine. A dorsal incision was made through the skin of the flank of the mouse, and the ovaries were removed. The ovaries were isolated by ligation of the most proximal portion of the oviduct before removal.
After 2 days of ovariectomy, some of the mice were injected intraperitoneally (100 μL/mouse) with vehicle (saline) alone or pregnant mare’s serum gonadotropin (PMSG; 5 unit/mouse) dissolved in saline. The others were injected subcutaneously (100 μL/mouse) with vehicle (sesame oil) alone, 17β-estradiol (E2; 300 ng/100 μL), or progesterone (P4; 2 mg/100 μL) dissolved in sesame oil.
The pituitary gland, oviduct, ovary, uterus and muscle were homogenized with RNA isoplus (TaKaRa Bio, Shiga, Japan). After chloroform extraction and isopropyl alcohol precipitation, RNA was dissolved in RNase-free DEPC (TaKaRa Bio) solution. The RNA concentrations were measured with the Nano-drop (Thermo Fisher Scientific Inc., Waltham, MA, USA). First strand cDNA synthesis was performed using the extracted RNA and oligo dT, followed by double-strand synthesis in RT buffer (Invitrogen, Carlsbad, CA, USA) with dNTP (BIO BASIC Inc., Ontario, Canada) and RTase (Invitrogen).
Conventional PCR was performed with 2X RbTaq™ PreMIX-Tenuto HOT (Enzynomics, Daejeon, Korea), template cDNA and each primer. Primers were designed for NUCB2 and β-actin on the basis of the mouse cDNA sequences. The following primer pairs were used NUCB2 forward 5’-TTTGAACACCTGAACCACCA-3’, reverse 5’-TGGTCTTCGTGCTTCCTCTT-3’ and 18S forward 5’-AGCCATGTACGTAGCCAT-3’, reverse 5’-ATCTTCATGGTGCTAGGAGC-3’ (Bioneer, Daejeon, Korea). The optimum temperature cycling protocol was used as 95°C for 15 s, 60°C for 30 s and 72°C for 30 min, using the Gene Pro thermal cycler (Bioer, China). The reaction products were run on a 2% agarose gel and visualized with ethidium bromide to check the length of the amplified cDNA.
Quantitative real-time PCR (qRT-PCR) was performed in buffer solution containing template cDNA, SYBR Green (Enzynomics), and each primer. Primer pairs were as follows; NUCB2 forward 5-AAAACCTTGGCCTGTCTGAA-3, reverse 5-CATCGATAGGAACAGCTTCCA-3 and 18 S forward 5’-GTCTGTGATGCCCTTAGATG-3’, reverse5’-AGCTTATGACCCGCACTTAC-3’(Bioneer). The optimum temperature cycling protocol was determined to be 95°C for 10 s, 60°C for 10 s and 72°C for 10 s using the Light Cycler 480 Real-time PCR System (Roche, Manheim, Germany).
Pituitary, oviduct, ovary, uterus and muscle were quickly removed and extracted the protein with RIPA lysis buffer (Rockland, NY, USA), the samples were SDS-PAGE and transferred to PVDF membrane. The membrane was treated in a blocking solution and incubated with rabbit anti-rat nesfatin-1 antibody (Phoenix Pharmaceuticals, Burlingame, CA, USA)/anti-mouse β-actin antibody (Santa Cruz Biotechnology, Dallas, DX, USA) followed by incubation with Goat anti-rabbit IgG (Bethyl laboratories, Montgomery, TX, USA) / Goat anti-mouse IgG (Bethyl laboratories), respectively. By Using the ECL Plus Western Blotting Detection Reagents (Amersham, UK), the membrane was detected to investigate the expression level of nesfatin-1 protein.
The tissues of oviduct were fixed in 4% paraformaldehyde buffer saline for 2 h. The tissues were rinsed in ethanol series to remove fixative residues, embedded in paraffin block. The tissue blocks were cut (10 μm sections) using a microtome, deparaffinized, and rehydrated with graded xylene-alcohol series, and then washed with PBS before immunostaining. The sections were incubated with rabbit anti-rat nesfatin-1 polyclonal antibody (Phoenix Pharmaceuticals), followed by incubation with Alexa fluor 488 conjugated goat anti-rabbit IgG (Bethyl laboratories). The sections were counterstained with DAPI (4’,6-diamidino-2-phenylindole; Sigma-Aldrich, St. Louis, MO, USA) for 10 min and mounted on the slides with mounting medium (Vector laboratories, Burlingame, CA, USA), and then observed under fluorescence microscopy (Axioskop2, Carl Zeiss, Germany).
RESULTS
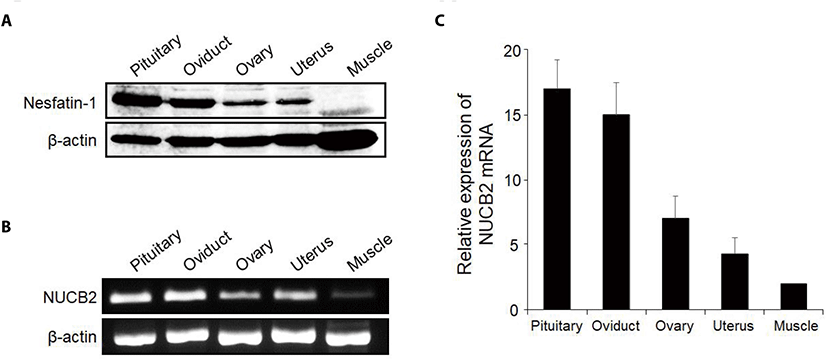
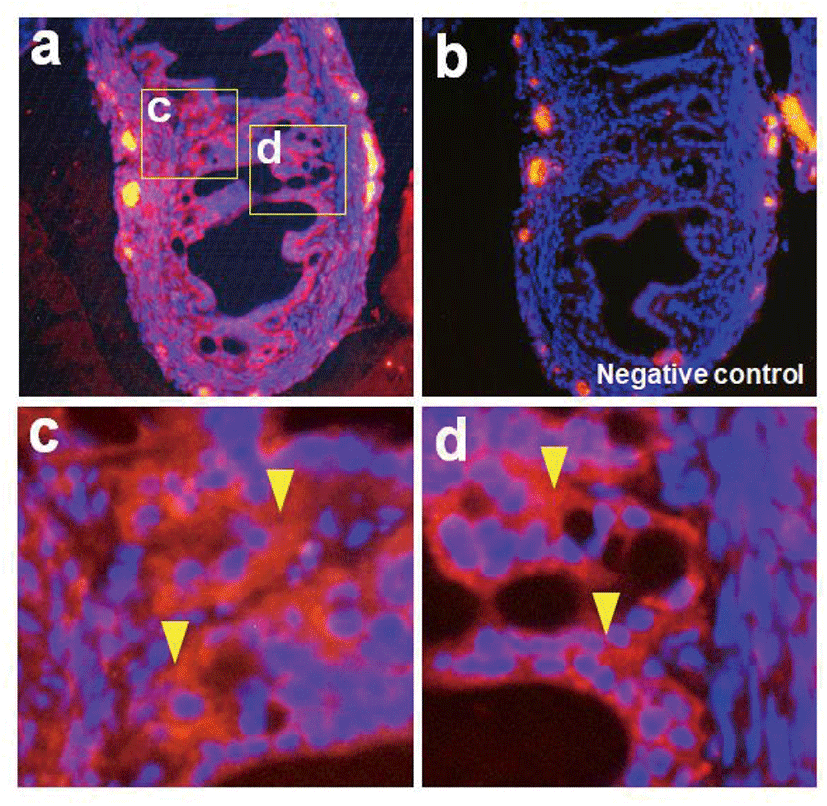
Nesfatin-1 protein was detected in the reproductive organs including the oviduct by western blotting. Interestingly, nesfatin-1 expression was as high in the oviduct as in the pituitary gland. The pituitary gland was used as a positive control, while the muscle was used as native control Fig. 1A). Similar to western blotting, NUCB2 mRNA expression was detected in the reproductive organs by conventional RT-PCR (Fig. 1B). As a result of qRT-PCR, NUCB2 mRNA expression levels in the oviduct were higher compared to ovary and uterus (Fig. 1C). Next, we investigated to nesfatin-1 localization in the oviduct using immunohistochemical staining with fluorescein conjugated nesfatin-1 antibody. As a result, nesfatin-1 were mostly positive in epithelial cells, while rare in muscle cells of the oviducts (Fig. 2).
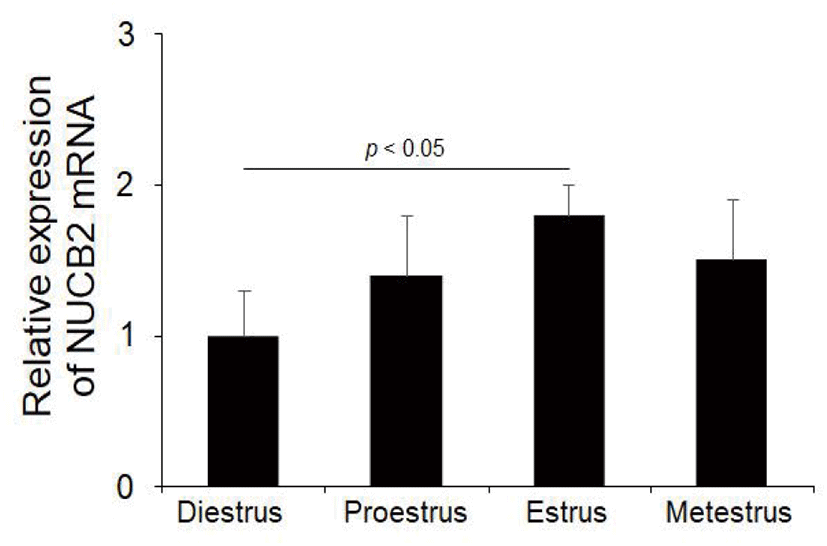
To determine whether NUCB2 mRNA expression in the oviduct is regulated through hypothalamus-pituitary-ovary axis, we measured the levels of NUCB2 mRNA during the estrus cycle. NUCB2 mRNA was expressed at higher in the estrus phase than in other phases during the estrus cycle (Fig. 3).
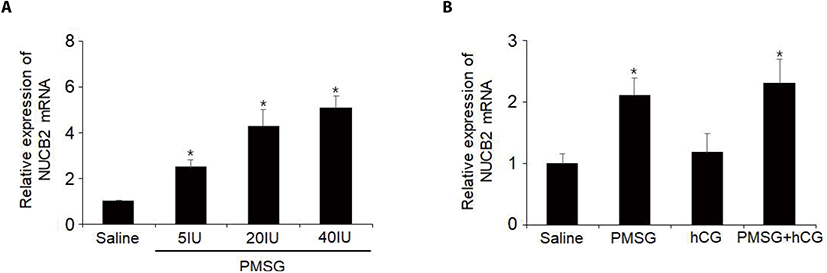
To examine whether NUCB2 mRNA expression in the oviduct is regulated by gonadotropin, we administered 5, 20, or 40 IU of PMSG to mice and measured the levels of NUCB2 mRNA in the oviductal tissues. The levels of NUCB2 mRNA were increased in a dose-dependent manner after PMSG injection (Fig. 4A). To elucidate whether hCG is involved in the effect of PMSG on NUCB2 mRNA expression, mice were administered together with PMSG and hCG. The levels of NUCB2 mRNA did not increase after hCG injection compared to saline treated controls. In addition, hCG injected with PMSG did not affect the increase in NUCB2 mRNA levels after PMSG administration (Fig. 4B).
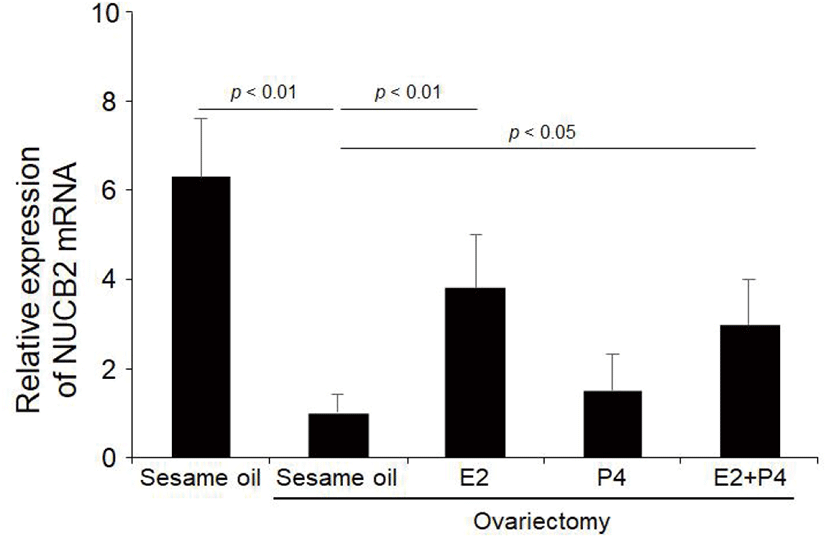
Next, to investigate if the increased levels of NUCB2 mRNA in the oviduct after PMSG injection is directly stimulated by gonadotropin or indirectly by sex steroid hormones, we injected 17β-estradiol and progesterone to ovariectomized mice. NUCB2 mRNA expression was significantly decreased in the oviduct after ovariectomy. On the other hand, NUCB2 mRNA expression was significantly increased in the oviduct of ovariectomized mice after 17β-estradiol injection, but not progesterone. However, NUCB2 mRNA levels were increased again after injection with both E2 and P4. (Fig. 5).
DISCUSSION
Nesfatin-1 known as an appetite-regulating hormone has been shown to be expressed not only in the brain but also in various organs, but the mechanism by which nesfatin-1 expression is regulated is not well known so far. In previous reports, we showed that NUCB2/nesfatin-1 is expressed in the reproductive organs of female and male mice and its expression is regulated by steroid hormones secreted by the gonads. As an extension of previous studies, we demonstrated here that NUCB2/nesfatin-1 is expressed in the oviduct of mouse and its expression is regulated by E2 secreted by the ovaries.
We first investigated whether NUCB2 mRNA and nesfatin-1 protein are expressed in the oviduct of mouse. Interestingly, NUCB2 mRNA and nesfatin-1 protein were expressed higher in the oviducts compared to the ovaries and uterus. In addition, nesfatin-1 protein was mostly localized in the epithelial cells of the oviducts, but rare in muscle cells. These results suggest that nesfatin-1 may be involved in oviductal function of mouse. Similar to this nesfatin-1, ghrelin as another well-known appetite-regulating hormone has also been reported to be expressed in the oviduct. In the oviduct of Holstein heifers, ghrelin and GHS-R1A, the functional ghrelin receptor, are expressed in the secretory cells of the columnar epithelium and the luminal side, which is localized in the ampulla and isthmus (Deaver et al., 2013). This ghrelin functions between the invading placenta and the endometrium, such as endometrial embryo receptivity that influences pre-implantation embryo development, and endometrial decidualization and placenta formation in early pregnancy (Luque et al., 2014). Leptin, another appetite-regulating hormone, is also produced by oviduct and endometrial epithelium in mouse. This leptin plays an integral role for early embryo development during early pregnancy in mouse oviduct (Kawamura et al., 2002; Kawamura et al., 2003). Leptin affects transport and maturation of the oocytes, spermatozoa, and embryos and fertilization in the oviduct of rats (Archanco et al., 2007). Leptin is also produced in the porcine oviduct for maturation of oocytes and preimplantation embryo development (Craig et al., 2005). Recent report showed that lepitn synthesized and secreted in the oviduct of brown frogs play an important role in autocrine or paracrine regulation in oviductal hypertrophy (Xi et al., 2017). Given these reposts, nesfatin-1 may also play an important role for sperm capacitation and early embryo development in the oviduct like other appetite-regulating hormones.
In addition to these appetite-related hormones, it has been reported that many types of proteins such as growth factors and cytokines are produced in the oviducts. One of the proteins secreted from the oviduct, oviduct-specific glycoprotein 1 (OVGP1), is synthesized in the oviductal epithelial cells and secreted into the oviductal lumen. OVGP1 is known to play an important role in fertilization and migration and early development of embryos (Rapisarda et al., 1993; O’Day-Bowman et al., 1996). Another physiological function of OVGP1 in the oviduct is to enhance the binding and penetration of sperm to the zona pellucida (ZP) of oocyte (O’Day-Bowman et al., 1996; Boatman et al., 1997). Glycodelin-A is a glycodelin called placental protein 14 (PP14) or progesterone associated endometrial protein (PEP). Glycodelin is also known to be synthesized in the oviduct, but to inhibit sperm binding to ZP of oocyte in contrast to OVGP1 (Chiu et al., 2007). These oviductal substances may be regulated through autocrine/paracrine mechanisms by nesfatin-1 produced in the oviducts. On the other hand, nesfatin-1 protein in the oviduct may be directly involved in providing a suitable environment for sperm capacitation and for the survival of fertilized oocytes.
Next, we investigated whether there is a change in the expression of NUCB2/nesfatin-1 in the oviduct during the estrus cycle. NUCB2 mRNA expression was higher in estrus stage than in other stages during the estrus cycle. This suggests that NUCB2 expression in the oviduct may be regulated by gonadotropins through hypothalamus-pituitary-ovary axis. It is well known that the estrus cycle is regulated by gonadotropins such as FSH and LH secreted from the pituitary gland. Therefore, to examine whether NUCB2 mRNA expression in the oviduct is regulated directly by gonadotropins, the levels of NUCB2 mRNA in the oviducts were measured after PMSG and hCG administration. NUCB2 mRNA levels increased in the mice injected with PMSG, but not with hCG. PMSG has activity like both FSH and LH, while hCG has activity only like LH. Given this, it seems that NUCB2 mRNA expression is stimulated by FSH, but not LH. In our previous study, we showed that administration of PMSG increased in dose-dependent manner NUCB2 mRNA expression in the ovary and uterus of mice (Kim et al., 2019).
To clarify whether the increase of NUCB2 mRNA levels after PMSG administration is due to injected PMSG or sex steroid hormones secreted by the ovaries that are stimulated by injected PMSG, we injected 17β-estradiol and progesterone to ovariectomized mice. NUCB2 mRNA expression was significantly decreased in the oviduct of ovariectomised mice, but increased again by 17β-estradiol injection rather than progesterone. These results showed that NUCB2 mRNA expression in the oviduct is regulated by 17β-estradiol secreted from the ovaries, but not by gonadotropin produced by the pituitary gland. Sex steroid hormones regulate production of various substances in luminal fluid of oviduct at different stages of the estrus cycle, which play an important role for gamete maturation and activation, fertilization and early embryo development (Lamy et al., 2016). In the bovine oviducts, E2 in follicular fluid upregulates expression of endothelin 2 (EDN2) which contracts the smooth muscles via GPER1 in the isthmic epithelial cells (Nishie et al., 2018). OVGP1 is secreted in large quantities in the preovulatory phase of the menstrual cycle in humans and is regulated by ovarian steroids. (Rapisarda et al., 1993; O’Day-Bowman et al., 1996). These results support that NUCB2 mRNA expression in the oviduct is also regulated by sex steroid hormones.
The present study demonstrated that NUCB2/nesfatin-1 is expressed in large amounts in mouse oviduct, and its expression is regulated by 17b-estradiol secreted from the ovary. These results suggest that nesfatin-1 can affect the oviductal function under ovarian control. Further research is needed on the specific function of nesfatin-1 in the oviducts.

