INTRODUCTION
It has been well established that photoperiod is a principal factor to control the reproductive functions of golden hamsters. Long photoperiod (LP, more than 12.5 h to 24 h in a day) always keeps the reproductive activities (Gaston & Menaker, 1967). While short photoperiod (SP, less than 12.5 h to complete darkness in a day) leads to total regression of testicles in 8 weeks of exposure in the laboratory facility (Elliott, 1976; Reiter, 1980; Choi, 1996; Choi & Lee, 2012). A demarcate period of time that the reproductive activities are divided into two different states has been known as 12.5 h in a day (Stetson & Watson-Whitmyre, 1984; Stetson & Watson-Whitmyre, 1986).
As other mammals used widely in the investigation of fertility, the reproductive activities of golden hamsters are also governed by gonadotropin releasing hormone (GnRH) (Pickard & Silverman, 1979; Choi & Lee, 2012; Choi, 2013). The hypothalamic-pituitary-gonadal (HPG) axis is responsible for initiation and maintenance of spermatogenetic activity in golden hamsters as well. GnRH is produced in hypothalamus and secreted to the pituitary through the hypothalamic-pituitary portal vessel, inducing the releases of gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH and LH act on the testes. In the testes, LH stimulates the Leydig cells located in interstitial tissues among seminiferous tubules and the cells then produce and secrete testosterone. Simultaneously, FSH supports the function of Sertoli cells, a mediator for effects of testosterone and exerts its effect on germ cells for successful spermatogenesis within the seminiferous tubules.
Gonadotropin FSH and LH levels in the blood have been reported to be reduced in the hamsters with regressed testes (Pickard & Silverman, 1979; Steger et al., 1982). The reduction of gonadotropins was interpreted due to the diminished secretion of GnRH from the hypothalamus. On the other hand, a reduced level of FSH and a sustained concentration of LH in the blood of the hamster with testicular regression were described (Kawazu et al., 2003). In the same report the both concentrations of the gonadotropins produced in the pituitary were decreased. The discrepancy in the concentrations of LH between pituitary and blood was attributed to the blood-collecting frequency in consideration of pulsatile release of hormones. But the results in the case of LH provokes a suspicion about regulatory mechanisms of synthesis of mRNA, production of proper hormones, and secretory system because the amount of hormone formed within the pituitary was not consistent with the level of hormone detected in the blood. In general, total concentrations of a hormone secreted in a day might be proportional to both the amplitude and frequency (Stetson & Watson-Whitmyre, 1986). The observations of low pituitary concentration and unchanged serum level of LH need to be more deeply examined.
The gonadotropin hormones are formed by two subunits, one common alpha subunit and another hormone-specific unique ß subunit. The hormone subunits are formed by the translation of each mRNA in the pituitary. Thus, there are three genes in the gonadotropin FSH and LH, whose chromosomal locations has not been known so far. But as mouse being used widely as well as human, the hamster gonadotropin genes might have been dispersed in separate chromones. The pituitary concentrations of the functional gonadotropins are dependent on the transcriptions, translations, and correct modifications of subunit polypeptide to form a combinational manufacture of proper polypeptide within the pituitary. Thus, the expressions of each subunit gene were needed to investigate in the pituitary.
Therefore, the goal of the present work was to show the expressions of the gonadotropin subunit genes related to reproductive states in testicles of the male golden hamsters whose reproductive activities were mediated by photoperiod. And the nucleotide and amino acid sequences of the genes were to identify and compare with the sequences reported previously.
MATERIALS AND METHODS
Male golden hamsters (Mesocricetus auratus) were used in this experiment. They were housed in the breeding boxes made by wooden boards and equipped with the photoperiodic lighting scheme by the electric timer. The lighting coming from the outside was completely blocked. The air was allowed to ventilate through the small light-proof fans in one side. The standard laboratory mouse chow and tap water were supplied to animals ad libitum. The sanitary conditions were managed on a daily basis. The schedule of animal management was approved by the Yong-In University Institutional Animal Care and Use Committee (YUIACUC-2020-03).
Two different photoperiods were applied to the breeding boxes. According to the photoperiods, the timers were set by long photoperiod (LP; lighting of 14 h and darkness of 10 h) or SP (lighting of 10 h and darkness of 14 h). The illumination of animal room outside the breeding boxes was consistent with the SP lighting condition. The ambient temperature was kept at 22±1°C. The mature male golden hamsters were divided into two groups. The animals were kept in LP or SP lighting condition for 8 weeks.
At the end of this 8 weeks experiment, the golden hamsters were decapitated and subjected to the bioassay. The weights of the reproductive organs, including testes, epididymides, and seminal vesicles, were measured. Also, some internal organs, that is, heart, liver, spleen, kidneys, and lungs were cut off and immediately weighed. The testicles were kept in 4% paraformaldehyde until the histological examination.
The trunk blood was collected into clean green tubes at the time of decapitation. The whole blood was kept at refrigerator (4°C) overnight and serum was collected by spinning down. The serum was maintained at freezer (−20°C) before use. After thawing the frozen serum, the concentrations of testosterone were measured by enzyme-linked immunosorbent assay according to the manufacturer’s manual (ELISA kit, Catalog No: E-EL-0155, Elabscience Biotechnology, Houston, TX, USA). The standard hormones were reconstituted with standard diluent at room temperature in the concentrations suggested by the manufacturer. The plate was subjected to the microplate reader and conducted measurement at 450 nm immediately.
The expressions of gonadotropins subunit genes were examined by reverse transcription polymerase chain reaction (RT-PCR) in the pituitary of two reproductively active and inactive golden hamsters. The genes applied for RT-PCT were, common gonadotropin alpha (CGa), follicle stimulating hormone beta (FSHβ), and luteinizing hormone beta (LHβ). The nucleotide and amino acids sequences of the genes were analyzed and compared with the sequences reported previously.
The primer sequences are shown in Table 1. Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used as reference standard for RT-PCRs in the present study. Sequence analyses were done by a commercial sequencing service company (Bioneer, Daejeon, Korea).
RT-PCR, reverse transcription polymerase chain reaction; bp, base pair; CGa, glycoprotein hormone alpha; F, forward, R, reverse; FSHβ, follicle stimulating hormone beta; LHβ. luteinizing hormone beta; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Total RNAs were isolated from testis samples using TRIzolⓇ Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. That is, the small pieces of testis (50-100 mg) were cut off and sonicated (VCX130, Vibra CellTM, Sonics & Materials, Newtown, CT, USA) with 1 mL of TRIzolⓇ Reagent. The samples were transferred to new clean microcentrifuge tubes and spun for 5 min at 13,000×g at 4°C. The supernatant was moved into the new tubes and kept for 5 min of incubation, allowing to dissociate the nucleoprotein complex. 0.2 mL of chloroform was added and capped firmly the tubes. Following the incubation of 2-3 min, the tubes were spun for 15 min at 13,000×g at 4°C. The upper aqueous phase was transferred to the clean tubes. 0.5 mL of isopropanol was added and kept at room temperature for 10 min. Then the tubes were spun for 10 min at 13,000×g at 4°C. The supernatant was removed and the pellets were resuspended in 1 mL of 75% ethyl alcohol. After agitation, the samples were spun for 5 min at 8,300×g at 4°C. The supernatant was eliminated and the pellets were dried for at least 5 min. The pellet was solubilized with 20-50 μL of RNase-free water. Quantitation of the RNA was measured by the absorbance at 260 nm and 280 nm.
The extracted total RNAs (tRNAs) were subjected to the RT-PCR reactions carried out with Maxime™ RT PreMix and AccuPower PCR Premix (Bioneer) according to the manufacturer’s instructions. Reverse transcription was principally performed to create complementary DNAs (cDNAs). The proper amount (1 pg-1 μg) of tRNA was relocated to clean microcentrifuge tubes and mixed with the following materials: reverse transcription reaction buffer, oligo (dT)20 primer, dNTPS (dATP, dTTP, dCTP, dGTP), reverse transcriptase, RNase inhibitor, and DEPC-treated water. The tubes were mildly agitated and incubated at 42°C for 60-90 min. The tubes were heated to 85°C for 5 min to inactivate the reverse transcriptase. The cDNA products were stored at −20°C.
PCR was carried out with the cDNA diluted with TE buffer (10 mM Tris [pH 8.0] and 0.1 mM EDTA). The microcentrifuge tubes with template cDNA (typically 10 ng) were blended with 10× PCR Buffer, dNTP Mix, primers (forward and reverse), Taq DNA Polymerase, 25 mM MgCl2, and water. The tubes were agitated gently and spun briefly to collect all components to the bottom of the tubes. The cycles of PCR were repeated 35 times with repeating the followings in the order: denaturing temperature of 94°C for 20 seconds, annealing temperature of 55°C for 30 seconds, and extension temperature of 72°C for 1 min. The final extension was completed at 72°C for 5 min and then cooled down to 4°C. The PCR reaction products were identified by gel electrophoresis in 1.0% agarose gel containing small amount of ethidium bromide (100 V, 40 min). The bands were examined using the image analysis system (Chemi Doc XRS, Bio-Rad, Hercules, CA, USA).
The PCR products were identified and purified through the agarose gel electrophoresis according to the manufacturer’s protocol (AccuPrepⓇ PCR/Gel Purification Kit, Bioneer). The visualized gel bands were cut off using sterile blade. The gel pieces were mixed with 3 volumes of FB buffer. The tubes with the gel pieces were incubated at 50°C for 10 min by inverting every 2-3 min. One volume of absolute isopropanol was added and blended immediately by inverting. The mixture was moved to a binding column in a 2 mL collection tube. The lid was caped and the tube was spun at 15,500×g for 1 min. The binding column was rebuilt with collection tube after removing the flow-through fluid. 500 μL of W2 buffer was added and spun at 15,500×g for 1 min. The binding column was rebuilt like above. Then the step with W2 buffer was repeated again and spun at 15,500×g for 1 min. The binding column tube was transferred to a clean 1.5 mL tube for elution. 30 μL of EA buffer was added carefully onto the binding column tube and kept at room temperature for at least 1 min. Finally, the new tube was spun at 15,500×g for 1 min. The eluant was sent to Bioneer to determine the nucleotide sequence of the genes.
The histological examinations of testicles were performed by using paraffin tissue section. The testicle tissues fixed in 4% paraformaldehyde solution were dehydrated in a series of increasing concentrations of ethanol (70%, 80%, 90%, 95%, and 100%) for 1.5 h with gentle shaking and immersed in absolute ethanol overnight. The tissues were submerged in xylene three times for 30 minutes and in paraffin at 56°C three times for 30 minutes. They were then embedded in paraffin and sliced at the width of 5 μm. The slices were mounted on slide glasses and the slides were subjected to hematoxylin (Sigma-Aldrich, St. Louis, MO, USA) and eosin (Sigma-Aldrich) staining solutions for 5 minutes, respectively. The slides were left to evaporate in the air for a while and treated with Canada balsam (Duksan Pure Chemicals, Ansan, Korea) for permanent specimen. They were observed under microscope (DM500, Leica, Wetzlar, Germany).
RESULTS
Various internal organs were isolated and weighed to inspect any weighable alterations of the organs at the end of experiment (Table 2). No significant changes were detected in the organs that were unrelated to the reproduction.
SP animals showed completely regressed testes, epididymides, and seminal vesicles.
Data are represented as the mean±SD (n=5).
LP, long photoperiod; SP, short photoperiod.
But distinct alterations were shown in the sexual organs. The testicles of animals housed in LP were big but those in SP very small, indicating a significant (p<0.05). Similar to the results of testicular weights, the weights of the epididymis and the seminal vesicle in the SP animals were significantly different to those in LP animals (p<0.05). All the animals housed in SP presented apparently miniatured reproductive accessory organs.
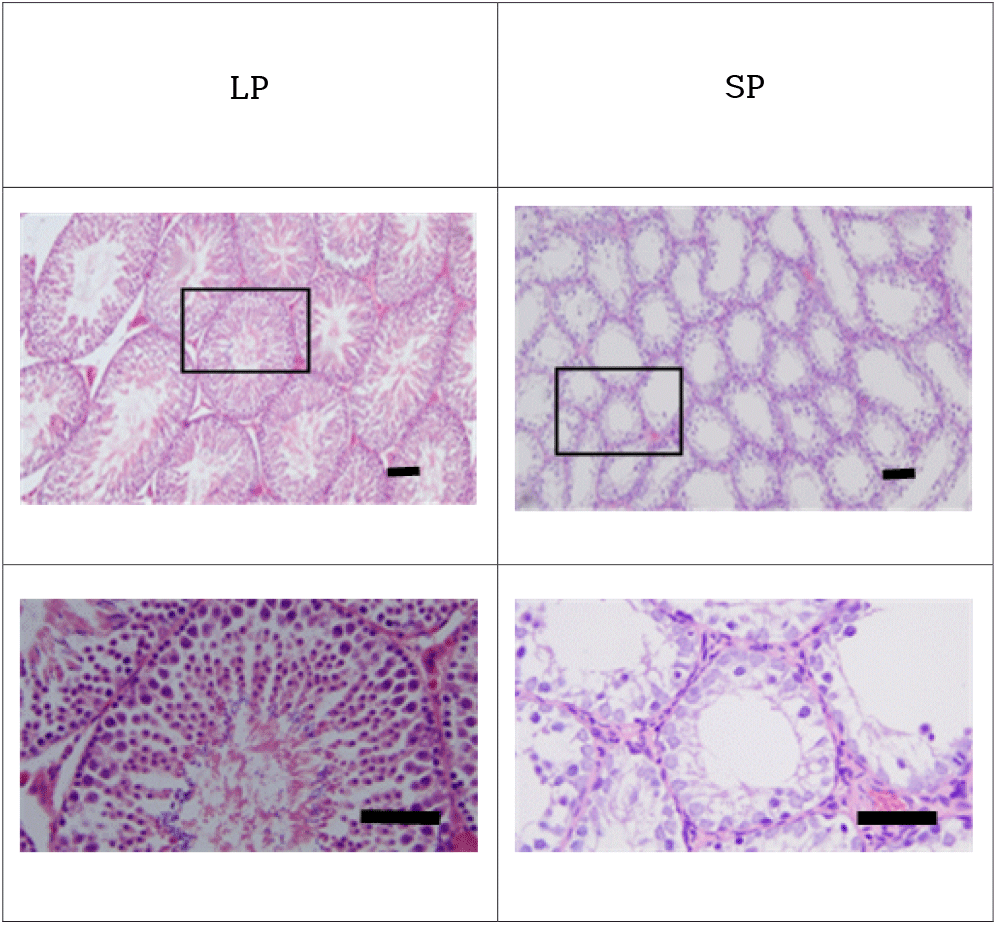
The representative histological examinations of the testes were represented in Fig. 1. The active testicles of animals housed in LP showed all stages of germ cells, including spermatogonia, spermatocytes, spermatids, and spermatozoa. These results were apparent in the spacious diameter and relatively thick epithelia of the seminiferous tubules. The lumen of the seminiferous tubules was full of spermatozoa with tails of wave-like pattern. Contrarily the inactive testicles of animals housed in SP displayed little germ cells in the epithelium of the seminiferous tubules. The spermatogonia and cells of initial stages of spermatogenesis were found without any spermatids and spermatozoa with tail in the seminiferous tubules of SP animals. The diameter of the tubules in SP animals was roughly less than half, meaning one eighth in volume.
Testosterone concentration measured in the blood of LP animals was 2.38±0.83 ng/mL and that in SP animals 0.77±0.33 ng/mL. The testosterone results showed significant difference between animals of LP or SP (p<0.05).
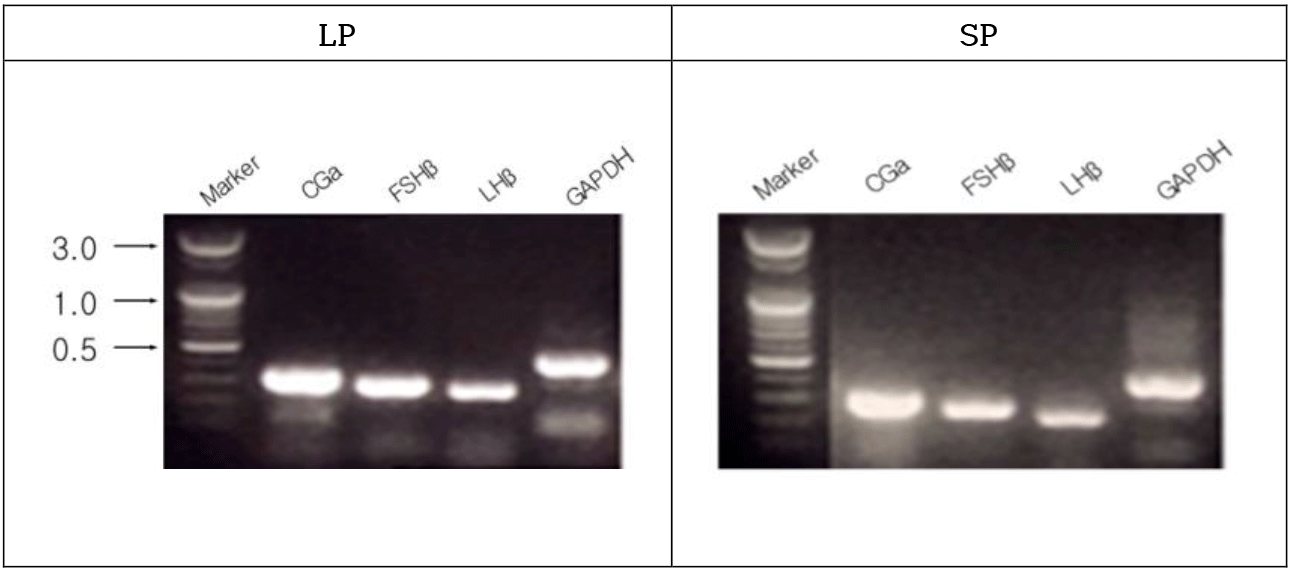
The male golden hamsters maintained in LP showed large testicles, representing full spermatogenesis. The gonadotropin hormone subunit CGa, FSHβ, and LHβ genes were expressed in the male golden hamsters housed in LP (Fig. 2). The genes were obviously expressed in the animals housed in SP as well. The animals housed in SP for 8 weeks showed very diminutive testicular masses, indicative of an arrest of spermatogenesis.
The 202 bases of CGa gonadotropin subunit mRNA were determined from the golden hamster. An unexpected result was that there was one base that was differently detected from the CGa sequence reported earlier (Fig. 3). The 138 and 192 bases were determined from the FSHß and LHß gonadotropin subunit mRNA of golden hamster, respectively. The sequences of both hormone-specific subunit genes were same as those reported previously. The sequence of bases of the gonadotropin CGa genes identified in this study was compared to the sequences of other rodents reported, including Chinese hamster, Siberian hamster, rat, and mouse (Fig. 3). The nucleotide sequence of CGa subunit of the golden hamster had homology of 94.6% (191/202) to Chinese hamster (Cricetulus griseus, NM_001246711.1), 92.6% (187/202) to Siberian hamster (Podopus sungorus, AB250761.1), 91.6% (185/202) to rat (Rattus norvegicus, V01253.1), and 91.6% (185/202) to mouse (Mus musculus, NM_009889.2).

The amino acids of CGa gonadotropin subunit were speculated from the sequence of mRNA identified in this investigation (Fig. 4). Sixty-seven amino acids were confirmed. One of them was different from the previous report where golden hamsters were also applied. In the present study the amino acid was determined as histidine but the earlier report showed serine in the place. The present amino acid sequence of CGa was same as the one reported previously in other animals, including Chinese hamster, Siberian hamster, mouse, and rat.

4. DISCUSSION
As documented definitely, the golden hamsters maintained in LP show large masses of testes, indicating fertile and functional spermatogenesis (Stetson & Watson-Whitmyre, 1984; Stetson & Watson-Whitmyre, 1986). As a seasonal breeding animal they present completely regressed testes in winter of natural environment or in 8 weeks exposed to SP in laboratory lighting scheme. The regressions are evidenced by diminished diameter of more than one half of seminiferous tubule (nearly one eighth in volume) without any distinct changes of inner organs as presented here, indicating the absence of germ cells (spermatozoa and spermatocytes) that results from the cessation of meiosis in the histological examination (Choi & Han, 2010; Choi & Lee, 2012; Jeon et al., 2021).
The reproductive activities of golden hamsters are also controlled by GnRH as other mammals (Pickard & Silverman, 1979; Jackson et al., 1984). For successful fertility, normal structure and accurate function of all parts of reproductive endocrine system are needed. A complex mechanism under the regulated function of the HPG axis is responsible for initiation and maintenance of spermatogenetic activity (Stetson & Watson-Whitmyre, 1984). In the report that both gonadotropins were reduced in the blood, the lowered level of FSH preceded the reduction of LH (Pickard & Silverman, 1979). FSH levels were definitely reduced but LH levels were somewhat disputable in blood in the reproductively suppression phase (Kawazu et al., 2003). In the report, the concentration of pituitary LH was significantly diminished at the time when the concentration of pituitary FSH was significantly lessened. The results that level of LH in the blood was not reduced could be due to infrequent blood-collection in the pulsatile release of hormones as the authors mentioned. Also, individual animal difference could be involved in the sustained level of LH in the blood due to the small numbers of animals. In other aspect using Siberian hamster when the animals were transferred to LP from SP, serum FSH began to increase within a week but serum LH concentrations were maintained at the lowered level for several weeks (Bernard et al., 2000). The gonadotropin hormone subunits detected in the pituitary showed same pattern where pituitary FSHß and common CGa amounts began to increase twice within a week. While pituitary LHß quantity was sustained at the lowered level of SP for several weeks. Thus, it is reasonable that gonadotropin releasing mechanism could be regulated differentially according to the hormones in the pituitary.
As gonadotropins are composed of two polypeptide subunits, there are three genes in the gonadotropin FSH and LH, whose chromosomal locations has not been known so far. But as mouse being used widely as well as human, the hamster gonadotropin genes might have been dispersed in separate chromones. The pituitary concentrations of the functional gonadotropins are dependent on the transcriptions, translations, and correct modifications to form a combinational manufacture of proper polypeptide within the pituitary.
All the three subunit mRNAs in the animals with entire regressed testes were expressed as much as the animals housed in LP, which were unexpected outcome. Not much information is available so far about degree of transcription and translation including modification of functional protein in the sexually inhibited hamsters. Similar observation to the present observations has been reported in the expression of GnRH in the hypothalamus (Brown et al., 2001). And in other report with immunocytochemical investigation, both animals housed in LP or SP showed same numbers of GnRH neurons (Urbanski et al., 1991). However, the perikaryal regions of GnRH neurons in the reproductively inactive hamsters became larger, speculating a suppression of GnRH secretion. The normal high state of transcription and low level of functional hormone in the pituitary present some alterations in the process of posttranscription. It could be decrease of translation activity. Or although the subunit mRNAs are translated, the combination of proper polypeptide subunits to form functional hormones could be impeded. Despite animals are experiencing the sexually quiescent period, it is speculated that the internal endocrine systems prepare for the translation and secreting system in the future (Bernard et al., 2000).
The nucleotide sequences of three gonadotropin subunits were examined in the range of RT-PCR performed in this investigation. Unanticipatedly it was found that one base in the CGa gonadotropin subunit mRNA was different from the report published previously (Suzuki et al., 2002). In comparison of the nucleotide sequence of CGa to other rodents including Chinese and Siberian hamsters, mouse, and rat, the base was consistent with the base that identified in this study. The other two FSHß and LHß gonadotropin subunit mRNA of golden hamster were same as those reported earlier.
The different nucleotide resulted in an altered amino acid, which was arginine. The earlier report showed serine in the place of arginine confirmed in this study (Suzuki et al., 2002). The amino acid presented here was same as the one reported previously in other animals as mentioned above.
In conclusion, the three subunit genes in testes of the sexually inactive animals were expressed as much as those of sexually active animals. All the nucleotide sequences of gonadotropin subunits identified in this study were same as those reported previously except for one base in CGa. An unsure amino acid deduced from the CGa sequence was confirmed as arginine that is different from the previous report. The results suggest that animals with regressed testes prepare for the sexually active period forthcoming in the future.

