INTRODUCTION
In most teleosts, final oocyte maturation is a prerequisite for ovulation. When final maturation is complete, ovulation begins and oocytes are released from the ovarian follicles. These processes are regulated by the teleost progestin, 17α, 20β-dihydroxy-4-pregnen-3-one (17α20βP), is one of the main maturation-inducing steroids (MIS; Goetz, 1983; Nagahama & Yamashita, 2008). In addition, prostaglandins (PGs), are synthesized from arachidonic acid (AA) by cyclooxygenase, also have important roles in ovarian maturation, ovulation, steroidogenesis, and sexual behavior (Goetz et al., 1991; Lister & Van Der Kraak, 2008; Chourasia & Joy, 2012). PGs induced germinal vesicle breakdown (GVBD) and ovulation in yellow perch, Perca flavescens (Goetz & Theofan, 1979) and in Salvelinus fontinalis (Goetz et al., 1982); they are strongly associated with the process of ovulation in fish (Lister & Van Der Kraak, 2008; Lister & Van Der Kraak, 2009).
There are several groups of PGs. In mammalian ovaries, prostaglandin E2 (PGE2) is involved in the process of ovulation of periovulatory follicles (Espey & Richards, 2006; Duffy, 2015), while prostaglandin F2α (PGF2α) plays an important role in the luteolytic events of post-ovulatory follicles (Sugimoto et al., 2015). Studies on many other teleost have explored the role of PGs in fish ovulation. PGF2α serves as a regulator of ovulation in many fish species studied to date. PGE2, which is an essential mediator for ovulation in mammals, plays a role in the ovulatory process in several teleost species (Fujimori et al., 2011; Fujimori et al., 2012). In yellow perch, oocyte ovulation suppressed in vitro by indomethacin (cyclooxygenase inhibitor) could be restarted by prostaglandin E1 (PGE1), PGE2, and PGF2α; and PGE2 was the most active among them (Goetz & Teofan, 1979). Moreover, 17α20βP significantly increased PGF2α levels in culture medium of the ovarian follicles with the time of ovulation (Goetz et al., 1989). Using Eurasian perch, Henrotte et al. (2011) reported that AA induced the production of 17α20βP, an effective steroid in the induction of oocyte maturation and ovulation in teleost, as well as the production of PGE2 and PGF2α. In addition, 17α20βP production was stimulated with exogenous PGE2.
The regulation of sex steroid and PG production in teleost ovaries during the breeding season is not fully understood. Using in vitro incubation methods, Kagawa et al. (2003) demonstrated 17α20βP induced ovulation through the synthesis of endogenous prostaglandin in the follicular layers of the Japanese eel, Anguilla japonica. Other in vitro studies in P. flavescens, 17α20βP was reported as a release inducer of prostaglandin E and F within mature follicles (Berndtson et al., 1989). Little is known about their mutual relationship.
Our previous studies reported that PGE2 possibly plays important roles in final oocyte maturation (GVBD) in vitro in the longchin goby, C. dolichognathus (Kim & Baek, 2017). This study focused on the effects of prostaglandins on 17α20βP production in vitro during the ovulation process in the longchin goby.
MATERIALS AND METHODS
The experimental fish (4.7–5.6 cm in body length) were collected at the coastal waters of Chongsapo, Busan, Korea, during the breeding period (March–April). The ovaries were taken from several mature females to obtain oocyte follicles (approximately 850–920 μm in diameter) at the migratory nucleus stage (Fig. 1A). After separating the ovaries into small pieces in ice-cold balanced salt solution (132.96 mM NaCl, 3.09 mM KCl, 0.28 mM MgSO4·7H2O, 0.98 mM MgCl2·6H2O, 3.40 mM CaCl2·6H2O, 3.65 mM HEPES), approximately 20–30 follicle-enclosed oocytes were incubated in each well of a 24-well culture plates containing 1 ml of Leibovitz L15 medium (Gibco) and/or 5–500 ng/ml PGs (PGE1, PGE2, and PGF2α; Sigma) and 17α20βP (Sigma). The plates were incubated for 16–18 h at 18℃ with constant gentle shaking. The pH and osmolality of the medium were adjusted to 7.7 and 300 milliosmoles, respectively.
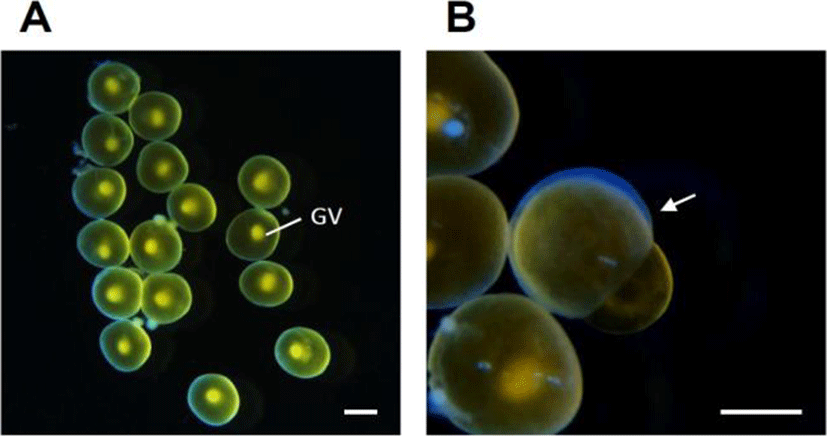
After incubation, oocytes were fixed with clearing solution (ethanol:formalin:glacial acetic acid=6:3:1). The number of oocytes that had completed final oocyte maturation (GVBD), or had ovulated (Fig. 1B), was counted in each well under low-power magnification using a dissecting microscope. Steroids from media were extracted twice using five volumes of ethylacetate: cyclohexane (1:1), dried under nitrogen gas and resuspended in phosphate buffer (pH=7.5). Then, 17α20βP levels were measured by radioimmunoassay (RIA) following Kobayashi et al. (1988). Radiolabeled 17α20βP was prepared from [3H]-17α-hydroxyprogesterone (Amersham Life Sciences) by enzymatic conversion as described by Scott's method (Scott et al., 1982).
All data are expressed as means±SEM, and SPSS software (version 21) for Windows was used for the Kruskal–Wallis test followed by Tuckey's test. A value of p<0.05 was considered statistically significant.
RESULTS
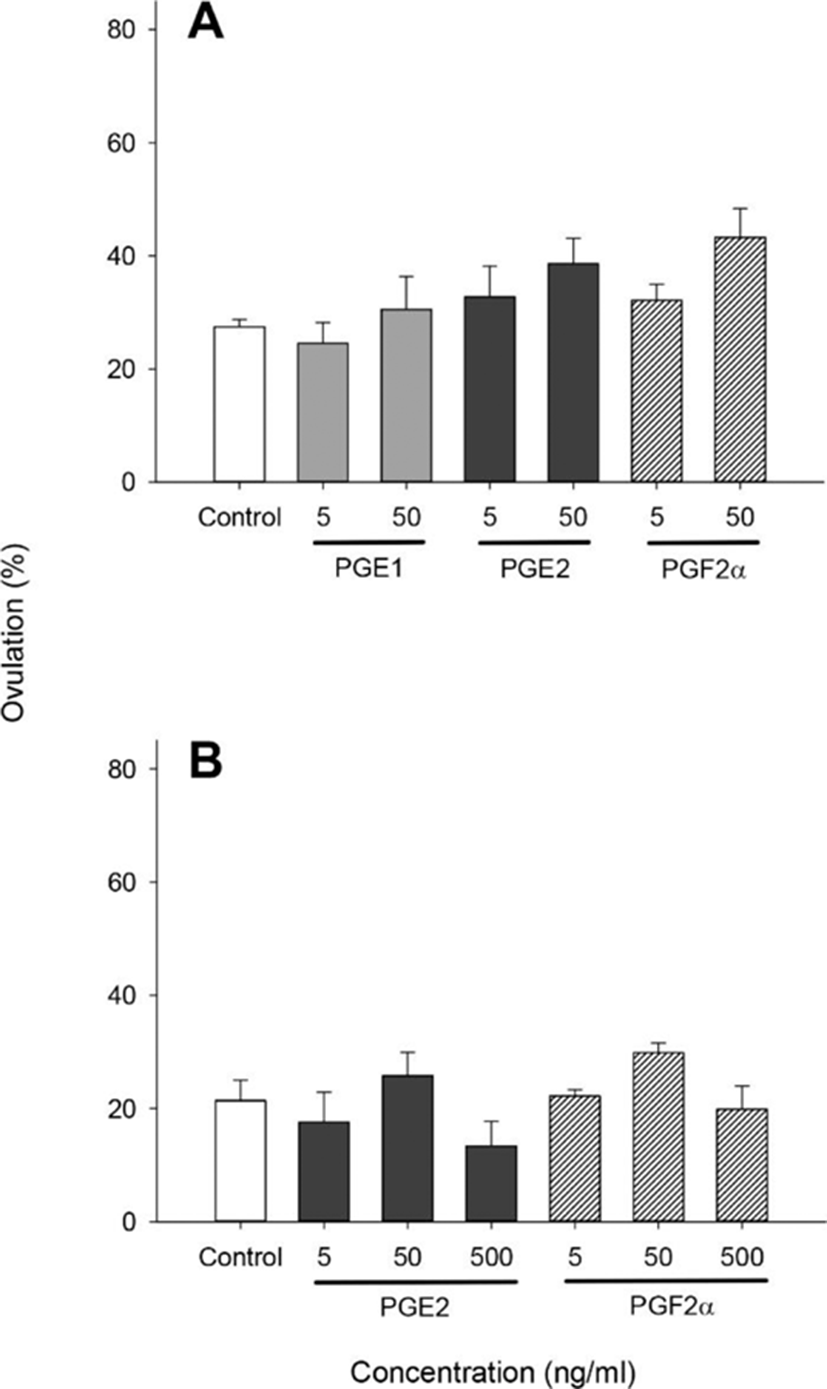
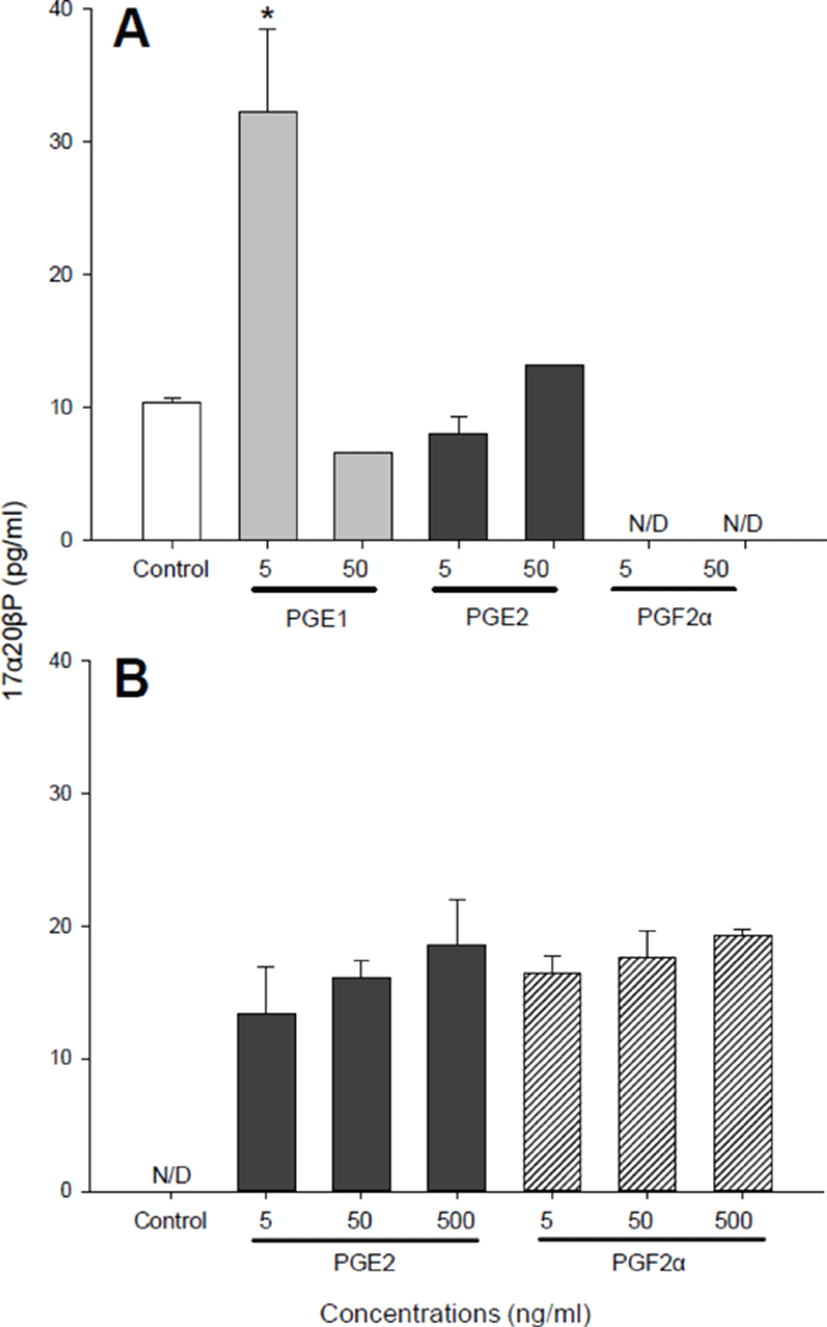
We performed several separate similar experiments using 850–920 μm diameter oocytes which were at the migratory nucleus stage. With the 890–920 μm oocytes (Fig. 2), no significant difference in ovulation was observed in any of the prostaglandins (PGE1, PGE2, and PGF2α) treated groups although PGE2 and PGF2α at concentrations of 50 ng/ml increased ovulation slightly (38.64±4.48 and 43.25±5.12%, respectively) compared with controls (27.47± 1.27%) (Fig. 2A). Then, we analyzed 17α20βP production by 890–920 μm oocytes (Fig. 3). Treatment with 5 ng/ml PGE1 alone resulted in a significant increase in the production of 17α20βP compared with controls (32.22± 6.25 vs. 10.33±0.42 pg/ml) (Fig. 3A). Whereas PGE2 and PGF2α in all concentration tested (5, 50, and 500 ng/ml) using the 890 μm oocytes resulted in increases in the production of 17α20βP compared with the controls, but there was no significant difference (Fig. 3B).
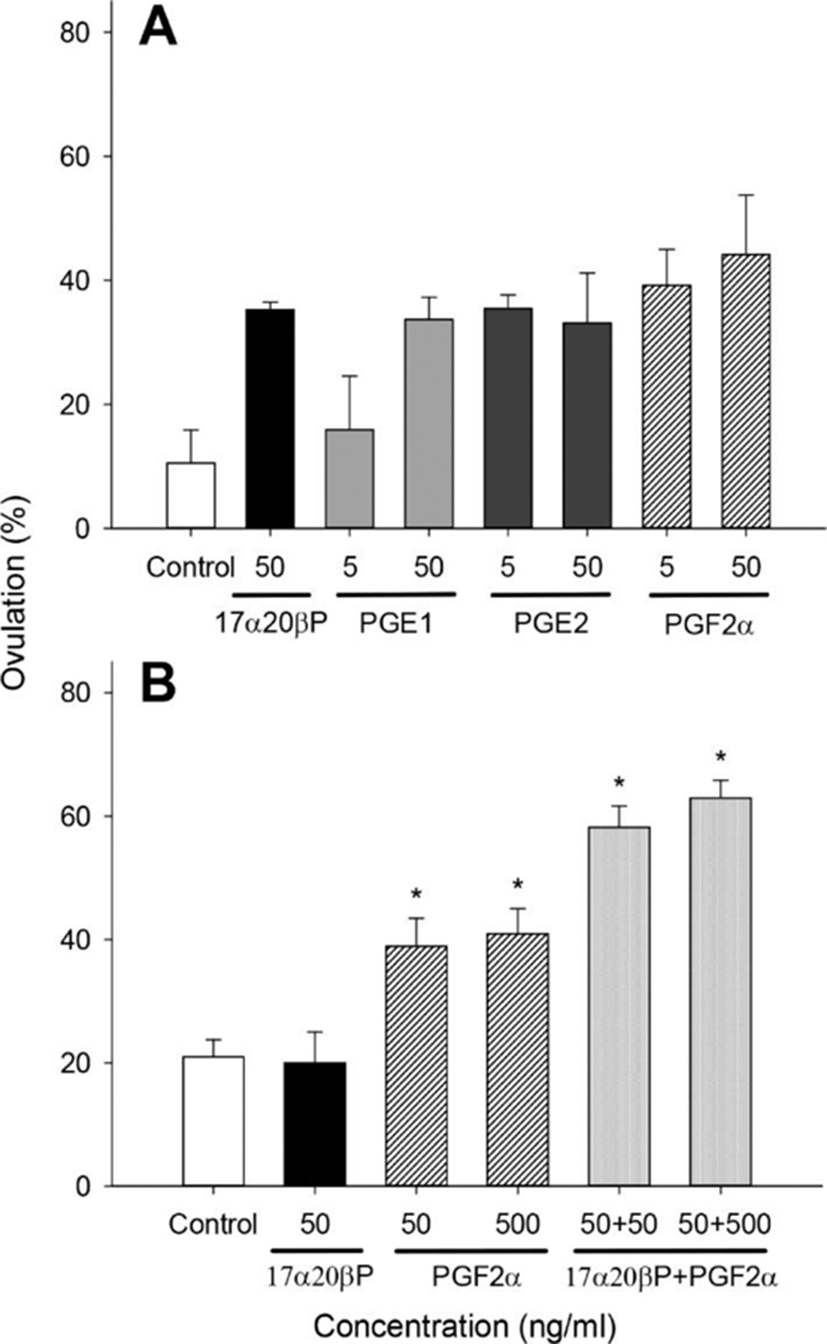
We examined the effects of 17α20βP and prostaglandins (PGE1, PGE2, and PGF2α) on ovulation using 850–900 μm oocytes (Fig. 4). No significant difference in ovulation was observed in any of the treatment groups, but it tended to increase in all tested groups except PGE1 5 ng/mL compared with controls in the 900 μm oocytes (Fig. 4A). We tested the effects of 17α20βP+PGF2α on ovulation at concentrations of 50 and/or 500 ng/mL using 850 μm. Treatment with 50 and 500 ng/mL PGF2α resulted in a significant increase in ovulation (38.90±4.54 and 40.94± 4.09%, respectively) compared with controls (21.00± 2.73%) (Fig. 4B). In addition, the combination groups of 17α20βP (50 ng/mL)+PGF2α (50 and 500 ng/mL) significantly increased ovulation (58.19±3.44 and 62.93± 2.86%, respectively) compared with controls.
DISCUSSION
Oocyte ovulation as well as maturation in teleost is induced by gonadotropins and MIS (Pinter & Thomas, 1999); especially, 17α20βP is one of the main MIS (Goetz, 1983; Nagahama & Yamashita, 2008). PGs play also an important role in maturation and ovulation of fish (Dongre et al., 2012). In different fish species, the ovulation of oocytes matured in the presence of gonadotropins or MIS can be induced in vitro by different PGs. Only PGF2α can induce oocyte ovulation in vitro in all fish species studied to date. Such induction was demonstrated in rainbow trout, goldfish, pike (Kagawa & Nagahama, 1981), Japanese eel (Kagawa et al., 2003), and Atlantic croaker (Patino et al., 2003).
Our previous studies demonstrated that PGE2 (50 ng/mL) significantly induced GVBD of matured oocytes (at the migratory nucleus stage) in vitro in the longchin goby, C. dolichognathus (Kim & Baek, 2017). In this study, we focused on the effects of PGs on ovulation and 17α20βP production by oocytes in vitro.
This studies showed that treatment with 50 ng/mL PGE2 and PGF2α increased ovulation slightly using 890–920 μm diameter oocytes although there was no significant difference; at the same time, 17α20βP production was stimulated with PGE1 alone at low concentrations (5 ng/mL), suggesting that these substances are related to the process of final maturation in the longchin goby, which 17α20βP was reported as major MIS (Baek, 2008; Baek et al., 2003).
In 850 μm oocytes, PGF2α at concentrations of 50 and 500 ng/mL resulted in a significant increase in ovulation. 17α20βP (50 ng/mL) alone had no observable effect on ovulation, but in the combined of PGF2α 50 or 500 ng/mL it caused the greatest effect on ovulation. The sensitivity of oocytes to the induction of ovulation varies between 850 and 890–920 μm, morphological maturation of these oocytes even at the migratory nucleus stage (Fig. 1); it appeared to vary depending on the migration status of nucleus (Baek et al., 2007).
In case of perch, it is possible that 17α20βP is regulating an enzyme in the follicles since PGF levels increased in the presence of 17α20βP while PGE levels appear to decrease (Goetz, 1997). In Eurasian perch, 17α20βP production was stimulated with exogenous PGE2 (Henrotte et al., 2011).
Skoblina (2009) reported that if the nucleus (germinal vesicle, GV) passed 2/3 of the distance to the animal pole, mature oocytes ovulated. At low 17α20βP concentrations, oocyte matured but not ovulated. However, their long-term exposure to low 17α20βP concentrations eventually induced ovulation although much later. Kagawa et al. (2003) demonstrated that 17α20βP itself can induce oocyte maturation and ovulation in vivo and in vitro. Among the prostaglandins, PGE2 and PGF2α induced in vitro ovulation in oocytes of the Japanese eel, but PGE1 was not effective. They concluded that PGF2α was the most effective in inducing ovulation of the Japanese eel. Moreover, the PGE group may not play a critical role in ovulation of the Japanese eel. These in vitro data are similar to our results. Similarly, PGEs (PGE1 and PGE2) have been shown to inhibit brook trout, S. fontinalis ovulation in vitro and follicle contraction (Goetz et al., 1982; Hsu & Goetz, 1992). Results from Joy & Singh (2013) showed that both PGF2α and PGE2 stimulated ovulation and that PGF2α was more potent in the catfish, Heteropneustes fossilis. Goetz & Cetta (1983) reported significant increases in plasma and ovarian prostaglandin F concentrations in naturally ovulating, compared with nonovulating, S. fontinalis.
When final maturation is complete, ovulation begins and these processes are regulated by hormones. 17α20βP is one of the main MIS (Nagahama & Yamashita, 2008); its precursors and metabolites as well as many other substances including PGs, also have important roles in final maturation and ovulation. For successful ovulation, a coordinated action of several proteolytic enzymes is also required (Ogiwara et al., 2015; Ogiwara & Takahashi, 2017). At present, from extensive studies, Takahashi et al. (2018) reported that it is worthwhile to examine how the PGE2 and PGF2α receptor systems contribute to teleost ovulation.
Very little is known about the association of PGs and a progestin, 17α20βP which has been identified as a main MIS in most teleosts including longchin goby. The elucidation of the relationship between 17α20βP and PGs synthesis in ovulating follicles needs further study.

