INTRODUCTION
Stem cells have been studied for replacing damaged cells or supporting regeneration of damaged tissue (Assmus et al., 2002; Wollert et al., 2004). Human embryonic stem cells (ESCs) have been evaluated as therapeutic cell sources due by their multi-lineage differentiation potential including neural cells (Reubinoff et al., 2000), blood cells (Kaufman et al., 2001) and pancreatic β -cells (D’Amour et al., 2006). However, clinical use of ESCs is faced with the ethical issue and the risk of teratoma formation (Kroon et al., 2008). The other cell source is induced pluripotent stem cells (iPSCs). Lately human iPSCs was created (Yamanaka, 2009; Thomson, 2012) through enforced expression of a limited number of genes critical to maintenance of pluripotency in ESCs. Similar to ESCs, iPSCs have also demonstrated their abilities of self-renewal and differentiation potential to all three germ layers (Sullivan et al., 2010; Burridge et al., 2011). However, using the exogeneous expression of four genes, including two pluripotency transcription factors (Oct4 and Sox2) and two protooncogenes (c-Myc and Klf4) (Takahashi et al., 2007), could raise a high risk of tumorigenesis when transplanted in vivo (Moriguchi et al., 2010).
Adult stem cells do not accompany an ethical issue and there is no report of the teratoma formation after transplantation in vivo. Previously, mesenchymal stem cells (MSCs) have been shown to proliferate multiple times in vitro and to differentiate into a variety of cell types of mesodermal lineage (Prockop, 1997). Later studies have also shown their differentiation potential into endodermal lineage such as hepatocytes (Wei et al., 2008), pancreatic β-cells (Kang et al., 2009), and ectodermal lineage including neurons (Woodbury et al., 2000). MSCs reside in various tissues including bone marrow, adipose tissue, umbilical cord, and amniotic membrane (Pittenger et al., 1999, Minguell et al., 2001). Although bone marrow-derived stem cells (BM-MSCs) are the most well-known and characterized MSCs, their retrieval from bone marrow accompany a highly invasive procedure. Therefore, many studies have been looking for alternative sources. Human eyelid adiposederived stem cells (HEACs), which could be obtained during plastic surgery, were successfully isolated and have shown the characteristics of neural crest cells. Moreover, they exhibited the typical characteristics of MSC, as revealed by differentiation into mesodermal lineages including adipocyte, osteoblast and chondrocyte, and an endodermal lineage such as insulin-secreting cells (Kang et al., 2009). Thus HEACs appear to be useful as cells sources for cell therapeutics.
FBS-containing medium has been the conventional medium for the isolation and cultivation of MSCs (Horwitz et al., 1999). However, using animal derivatives might bring a risk of contamination with bacteriophage, infectious bovine rhinotracheitis, parainfluenza-3, bovine viral diarrhea virus (Erickson et al., 1991), bacteria, prions, nanobacteria, yeast and endotoxins. A single injection of 108 hMSCs grown under 20% FCS would carry with it approximately 7 to 30 mg of calf serum proteins which might cause immune response to FBS in human patients when they were transplanted (Spees et al., 2004). Therefore, researchers are searching for alternative sources for human cell cultivation instead of FBS. Recent studies have suggested human body fluids including autologous serum (autoHS) (Yamamoto et al., 2003; Stute et al., 2004), allogeneic human serum (alloHS) (Le Blanc et al., 2007; Tateishi et al., 2008) umbilical cord blood serum (CBS) (Ma et al., 2012; Tekkatte et al., 2012) and platelet derivatives (Kocaoemer et al., 2007; Bieback et al., 2009), follicular fluid (HFF) (Qui et al., 2012) could be promising substitutes. Yet, there have been controversial issues about the advantages of HS, and detailed studies about the effects of human serum on stem cells have not been done.
For the first time, the present study revealed that human adult stem cells could move and form aggregates in vitro by the treatment with alloHS. Interestingly, the induction of the aggregation appears be specific to the human body fluids. And the change of expression of cytoskeleton-related genes is observed at HEACs cultivated with 10% HS.
MATERIALS & METHODS
HS was obtained from whole-blood donations of 6 female Asian donors. Blood was drained into sterile 50 ml tube (SPL, Korea) without anticoagulants and allowed to clot overnight at 4°C. They were centrifuged at 3,000 rpm for 30 min, and the supernatant was centrifuged again at 3,000 rpm for 30 min to remove any remaining particles. The six allogeneic human sera were pooled and sterile-filtered through 0.2-μm pores (Millipore, Billerica, MA). After inactivation at 56°C for 30 min, HS was aliquoted into 15 ml tube and stored at –20°C until use.
The human eyelid adipose tissue was obtained from five patients aged between 20 and 56 years (mean age, 39.6±5.9 years) undergoing cosmetic surgery with informed consent. They were washed with Dulbecco’s phosphate buffered saline (DPBS, Gibco, Grand Island, MA) and treated with 0.075% type I collagenase (Gibco) in DPBS for 30 min at 37 with °C gentle stirring. The supernatant was discarded and mixed with an equal volume of Dulbecco’s modified Eagle’s medium - low glucose (DMEM-LG, Gibco) supplemented with 10% FBS (Gibco) to neutralize the enzymatic activity. After centrifugation at 3,000 rpm for 10 min, the supernatant was removed and the remaining cell suspension was washed several times with DMEM-LG, and were plated in 25-cm2 culture flask (Nunc, Rochester, NY) containing DMEM-LG supplemented with 100 U/ml penicillin (Gibco), 0,1 mg/ml streptomycin (Gibco) 3.7 mg/ml sodium bicarbonate, and 10% FBS. The medium was changed twice a week. Passage 4 HEACs were tested in this study.
HEACs were seeded in 12-well plate (Costar) at density of 1,000 cells/cm2. Cells were cultivated with DMEM-LG containing 10% of HS, CBS, FBS, bovine serum (BS, Gibco), 15% Human follicular fluid (HFF), 5% HS –5% FBS and 0.4% of bovine serum albumin (BSA). Morphology of cells was observed during 18 days of culture. To investigate the timing and number of cells at the beginning chemotactical aggregation, cells were plated on a 12-well dish at a density of 102, 103, 104 or 105 cells/well. The number of cells was counted on the day of beginning of the aggregation. CBS and HFF were kindly donated from CHA Gangnam Medical Center with informed consent.
Total RNA was isolated using Tri-reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The concentration and quality of isolated RNA was determined using Nanodrop 2000 spectrophotometer (Thermo fisher scientific, Rockford, IL). The purity of RNA was assessed by determining the ratio of absorbance at 260 nm to that at 280 nm (>1.8). RT-PCR was carried out using a Techne PCR system TC-100 (Bibby Scientific Ltd., UK). Total 7.5 μg (1 μg /μl) RNA was reverse-transcribed using the following RT mixture: 20 mM MgCl2 (BioBasic Inc, Canada), 10×PCR buffer (BioBasic Inc), 10 mM dNTPs mixture (BioBasic Inc), 0.5 mg/ml oligo (d)T15 (Bionics), 40 U/ μl RNase inhibitor (BioBasic Inc) and 200 U/ μl AMV-RT (Invitrogen). RT reaction was allowed to occur for 60 min at 37°C, and the resultant cDNA subjected to PCR amplification.
For the conventional PCR, cDNA were amplified using the following PCR mixture: 25 mM MgCl2, 10×PCR buffer, 5 U/ μl Taq polymerase, 2.5 mM dNTP, 10 μM sense and antisense primer. Amplification was performed for 35 cycles at a denaturing temperature of 94°C for 30 sec and an extension temperature of 72°C for 30 sec. Annealing temperature was set depending on the species of primer. The PCR products were mixed with 6×lading buffer (0.25% bromophenol blue, 0.25% Xylene cyanol and 40% sucrose) and separated on 3% agarose gels. After electrophoresis, gels were stained with ethidium bromide. DNA signals on the gels were imaged under ultraviolet light using a Bioprofile image analysis system (Vilber Lourmat, Germany). Primers used in this experiment were shown in Table 1. All PCR mixture components were purchased from Takara. HEACs cultivated with 10% FBS were named HEACs, and HEACs cultivated with 10% HS were named agg-HEACs in this study.
All reagents and chemicals were purchased from the Sigma unless otherwise designated.
RESULTS
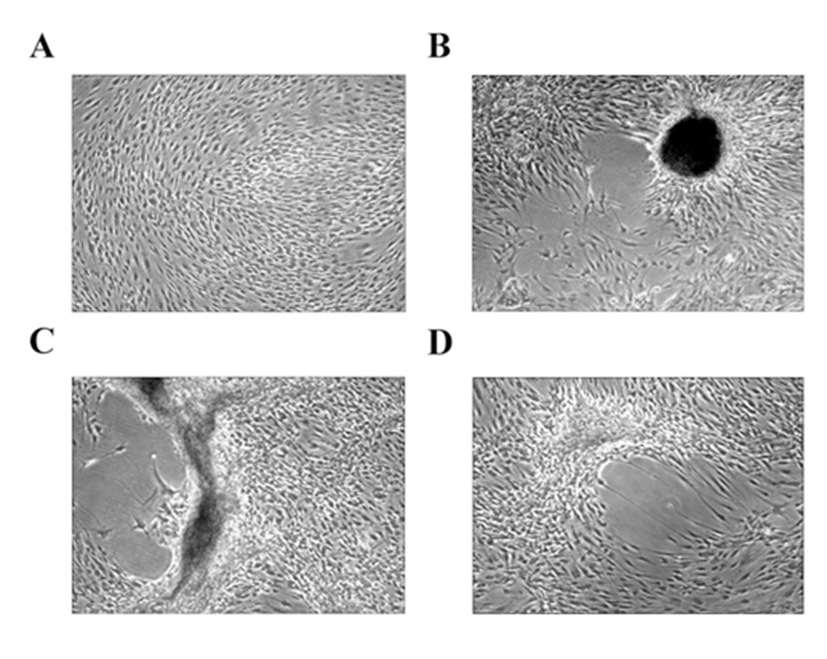
HEACs were isolated and proliferated in DMEM-LG supplemented with 10% FBS until passage 3. At passage 4, HEACs were transferred to a medium containing 10% HS in a 12-well dish for 3 weeks without subculture even though they reached 100% confluence. In FBS-containing medium, HEACs did not show any morphological change even after culture for 11 days (Fig. 1A). However, as shown in Fig. 1B, C, D, when they were cultivated in 10% HS-containing medium, HEACs exhibited a movement which directed to several spots after 11 days of culture. During further culture, migrated cells began to aggregate to form several mass which looked like egg-shape (Fig. 1B), C-shape (Fig. 1C) or simple cell clump-shape (Fig. 1D).
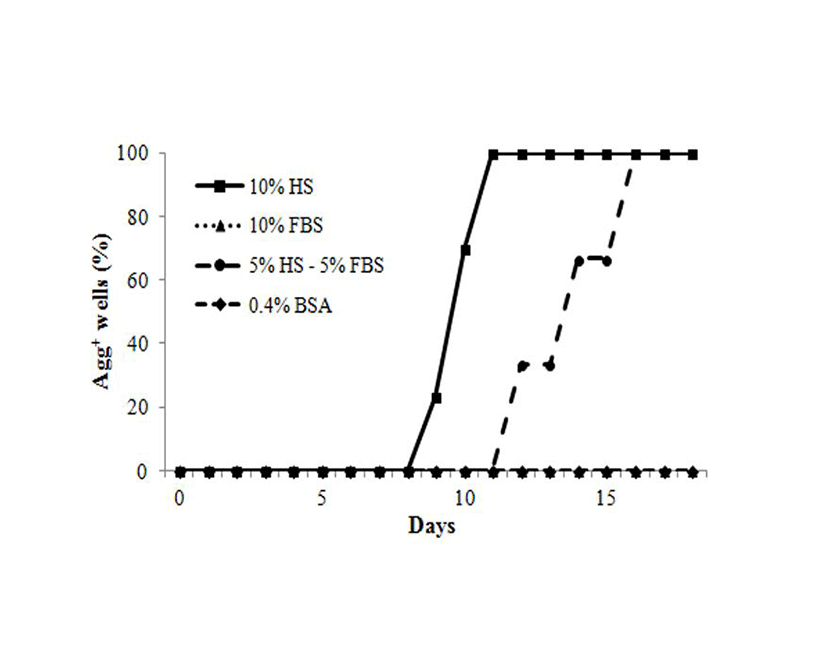
To examine whether all cells have the same response to HS different donor cells and other medium were tested. Aggregation was not observed in 10% FBS or 0.4% BSA-containing medium. However, 10% HS, and 5% HS plus 5% FBS induced aggregations of HEACs after 9 days of culture for HS and 12 days for 5% HS plus 5% FBS (Fig. 2). As early as 9 days following treatment, HEACs started moving to several-specific spots and formed aggregations. Between 9 and 11 days aggregates were observed responded to HS. In the other hands, less HS plus FBS-containing medium also induced aggregation, but starting date was delayed from 9-day to 12-day. These results are showing that HS might act as activator and FBS might act as an inhibitor.
To examine if there is any relationship between cell density and the treatment period, and aggregation of HEACs, cells were seeded at varying densities of 102, 103, 104 or 105 cells per well of a 12-well dish. As shown in Fig. 3A, aggregation started after culture for 16.0±1.6 days in 102/well, 12.3±1.3 days in103/well, 10.0 ±0.4 days in 103/well, and 9.0±0.4 days in 105/well. The results show that as the number of seeded cells increase, the aggregation form sooner (p<0.05). To see the exact number of cells at the time of aggregation, cells were harvested from each group on the day the aggregation started and each cell number was counted. When initial cell density was 103, 104 or 105, their total cell numbers at the time of aggregation were 24.0±1.8, 24.3±1.2 and 29.3±2.8×104, respectively (Fig. 3B).
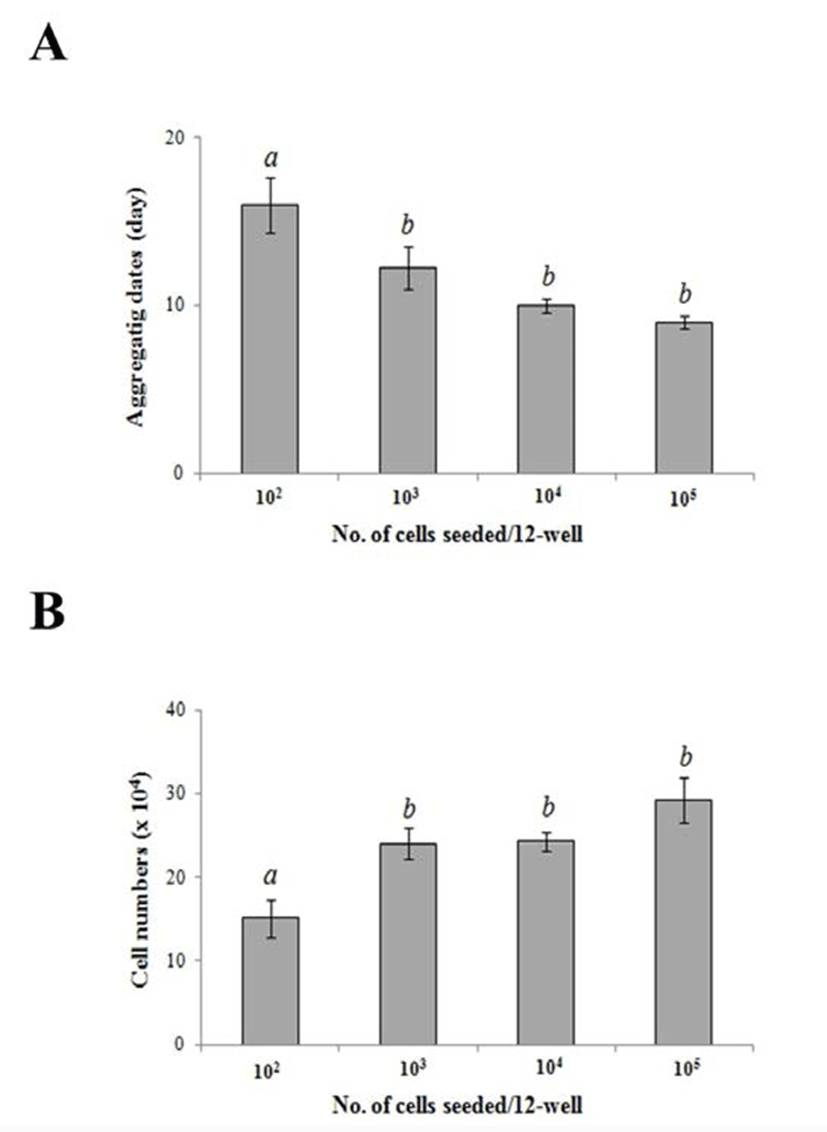
There was no significant difference among these conditions. However, when the initial cell density was 102/well, then the total cell number was 15.1±0.2×104, which was significantly smaller to the other group (p<0.05). These results show that HEACs cultivated for 9 days at a density of 15.1×104 cells.
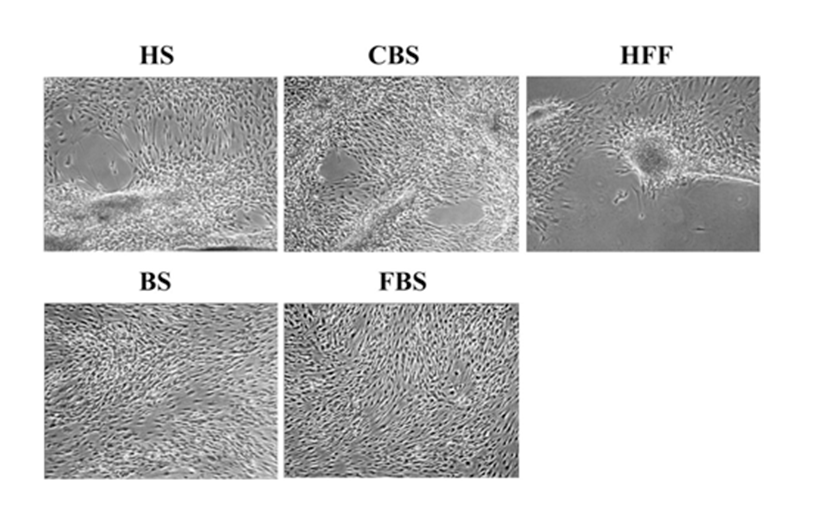
To investigate whether the aggregation is human body fluid-specific phenomenon, 10% of CBS, BS, FBS or 15% HFF were tested. Cells were seeded at density of 3.5×103cells/cm2 and treated for 18 days. When cells were cultivated with medium containing 10% HS and CBS or 15% HFF, cells began to move into several spots and aggregated, but not in 10% FBS or BS (Fig. 4). Average aggregating periods were 10.2± 0.1 days in HS-containing medium, 13.2±0.6 days in CBS-containing medium and 16.4±0.8 days in HFF-containing medium. These results indicate that chemotactic aggregation of HEACs could be induced not only by HS but also by CBS or HFF.
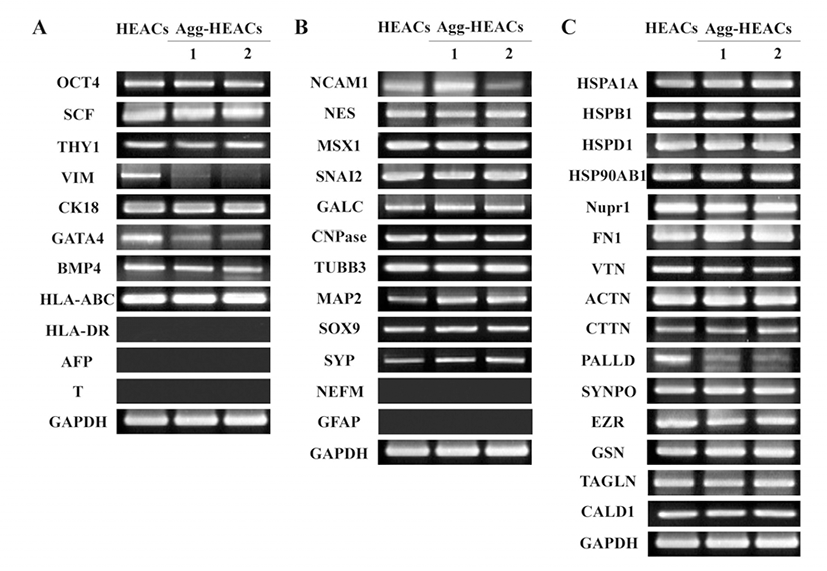
To investigate the effect of aggregation phenomenon on the characteristics of HEACS, expression of genes related with stem cells, stress, and cytoskeleton was examined. Agg-HEACs induced with 10% HS were examined their gene expression and it was compared with HEACs cultivated with 10% FBS. It was shown that agg-HEACs express consistently stem cell-related genes of OCT4, SCF and THY1, mesoderm/endoderm-related genes of CK18, endoderm-related genes of BMP4 (Fig. 5A) but not express endoderm-specific AFP and mesoderm-specific Brachyury (T). Immunerelated gene of HLA-ABC was expressed in agg-HEACs but not HLA-DR. There was no difference in expression level between HEACs and agg-HEACs. However, interestingly, agg-HEACs did not express VIM, and showed lower expression level of GATA4 gene compared to HEACs cultivated in FBS-containing medium. Previously, we reported that HEACs have neural-crest properties (Kang et al., 2009), therefore in this study we confirmed the expression of neural crest-related genes at both HEACs and agg-HEACs. The results showed that both HEACs and agg-HEACs express ectoderm- or neural progenitor-related genes of NCAM1 and NES, neural crest-related genes of MSX1, SNAI2, and glial cell-related genes of GALC and CNPase, neuron-related genes of TUBB3 and SOX9, but they do not express both GFAP and NEFM genes, related with glial and neuron cells, respectively (Fig. 5B). However, it was found that HS-induced aggregated HEACs increase mRNA expression of neuronrelated genes of MAP2 and SYP. On the other hands, to examine whether aggregation phenomenon involve the cellular stress, we investigated the expression of stress-related genes. The results showed that the expression of stress-related genes of HSPA1A, HSPB1, HSPD1, HSP90AB1 and Nupr1of agg-HEACs does not changed when it compared with HEACs cultivated with 10% FBS (Fig. 5C). Finally, to define the expression of ECM- and actin-related genes on agg-HEACs, mRNA expression was investigated. It was observed that both HEACs and agg-HEACs express ECM-related genes of FN1 and VTN and actin-related genes of ACTN, CTTN, SYNPO, EZR, GSN, TAGLN, and CALD1 with no significant difference in expression levels while the expression of actin-related gene of PALLD was markedly decreased in agg-HEACs.
DISCUSSION
In this study we found that HEACs move and form the aggregate by the treatment of HS in vitro and the aggregation of HEACs is specifically induced by the human body fluids.
Many researchers have extensively studied the HS as FBS-substitute for expansion of MSCs. For example, it is reported that autoHS shows similar effect with FBS on characteristics of MSCs including both expansion and multi-differentiation potential into adipocyte and osteoblast (Stute et al., 2004). Some reports showed that autoHS is better than FBS in proliferation capacity of BM-MSCs (Shahdadfar et al., 2005). These results suggest that autoHS might be a suitable candidate for cultivation of MSCs. However, it is practically difficult to apply for cell therapy since a large amount of auto-HS should be obtained from a single donor for cultivation of cells for transplantation. Therefore, it has been suggested that alloHS can be alternatively used for cultivation of MSCs as a feasible supplement of FBS because it can be easily obtained (Le Blanc et al., 2007; Kocaoemer et al., 2007). However, there has been a lot of controversy about alloHS for cultivation of MSCs. It has been shown that MSCs including umbilical cord-derived stem cells (UCSCs) have high proliferative potential in presence of alloHS. It showed that alloHS support a homogenous morphology and a stable expression of stem cells-related genes during long-term expansion (Hatlapatka et al., 2011). On the other hands, it has been reported that cells cultivated with alloHS enter senescence earlier than cells cultured in presence of FBS although it shows a much higher cumulative population doubling numbers until passage 6 (Kocaoemer et al., 2007). Also, some researchers argued that commercial alloHS can lead poor cellular yields, a notable cell death and morphological deterioration (Spees et al., 2004).
CBS, another human body fluid, can be used for FBS-substitute for cultivation of MSCs as a safe and unique humanized culture condition. It has been reported that CBS support to enhanced self-renewal capacity and osteogenic potential of MSCs (Jung et al., 2009). Interestingly, it showed that MSCs cultured with CBS was detached from the flask at passage 3, although cells showed high replicative ability until passage 2, whereas cells cultured in presence of FBS were continued to grow up as a monolayer (Shetty et al., 2007). This report supports our aggregation phenomenon of HEACs which move and form the cell aggregation in culture medium containing 10% CBS. Follicular fluid (FF) is a liquid that surrounds the oocyte with the attached follicular cells. It is well known that FF contains various biological active proteins that affect follicular growth and maturation (Sun et al., 2011). Recently, it is reported that the FF promote the proliferation of goat umbilical cord-derived MSCs (UCMSCs). On the other hands, higher concentration of follicular fluid (10 %) showed the induction of UCMSCs into oocyte-like cells.
In this study, we observed that HEACs form aggregates in vitro by the cultivation of human body fluids including alloHS, CBS, and HFF. Until now, there are not any reports about the aggregates formation of MSCs by allogeneic HS, CBS and HFF. They could not be observed this phenomenon because subculture of cells is usually performed before cells reach the confluence on the flask. In this study, we found that HEACs form the aggregation after the confluence by alloHS, CBS, and HFF but not by FBS and BS. It showed that it takes 4 5 days to aggregate after confluence in culture medium containing 10% alloHS and at least 9 days from initial cultivation at seeding density of 2.6×104 cells/cm2. When HEACs are cultivated with CBS or HFF, the culture period for the formation of aggregate of HEACs increased than allo-HS. From these results, we suggest that human follicular fluid including HS, CBS and HFF have a special factor to induce the aggregation of cells. Since the aggregation was not induced by FBS and BS, further studies should be performed to investigate whether this phenomenon is only observed with human body fluids. It can be disclosed using other animal fluids such as mouse and rabbit. Furthermore, it needed to be examined whether the aggregates formation of cells is only occurred at human stem cells or not.
To investigate the effect of aggregation phenomenon on the characteristics of HEACS, expression of genes related with stem cells, stress, and cytoskeleton was examined. As a result, agg-HEACs induced by alloHS showed remarkably low expression level of VIM gene, stem cell-related marker gene. Vimentin is a type III intermediate filament (IF) and it is known as a major cytoskeletal component of mesenchymal cells (Chang & Goldman, 2004). In addition, the results showed that expression of MAP2 and SYP, neural crest-related genes, increase on agg-HEACs compared to HEACs. MAP2 serves to stabilize microtubules (MT) growth by crosslinking MT with intermediate filaments and other MT. It is known that the increase of MAP2 is observed at the neuron which exhibiting a rapid neurite extension (Bogoch & Linial, 2007). These results are consistent with the findings of down-regulated expression of PALLD gene, a component of actin-containing microfilaments. In recent study, it has been found that downregulation of palladin by small hairpin RNA (shRNA) enhances invasive migration, indicating that the lower expression of palladin helps the cell migration (Chin & Toker, 2010). As the internal cytoskeleton of eukaryotic cells is composed of actin microfilaments, microtubules, and intermediate filaments, our results revealed that all three components of internal cytoskeleton are changed by the aggregation of cells. Taken together, our study demonstrates that agg-HEACs form with the change of expression of various cytoskeleton-related genes.
It is known that various environments such as temperature shock, oxygen shock, nutrient deprivation, and confluence cause the stress to the cells (Richter et al., 2010; Majmundar et al., 2010; Sengupta et al., 2010). To examine whether an aggregation structure can be an external factor of the stress, expression of stress-related genes including HSPA1A, HSPB1, HSPD1, HSP90AB1 and Nupr1 were examined in agg-HEACs. As shown in Fig. 5C, there were no differences of gene expression in both HEACs and agg-HEACs. This result suggests that stress might not be influenced when HEACs were cultivated with 10% HS. These are consistent with previous reports that demonstrated a stable phenotype of MSCs without the cell density (Haack-Sørensen et al., 2012).
In summary, these results demonstrated the movements and aggregation of HEACs by human body fluids, including HS, CBS and HFF at the first time. And it revealed that the expression of cytoskeleton-related genes is changed by cultivating with HS.

