INTRODUCTION
The interaction between neighboring cells present in a multicellular tissue is chiefly achieved by three junctions, including tight junction, adherens junction, and gap junction (Lawrence et al., 1978). Of these junctions, gap junction involves in the direct cell-cell communication through the reciprocal exchange of materials, such as signaling molecules, ions, and small metabolites, between cells (Lawrence et al., 1978; Valiunas et al., 2005). A complex of six connexin (Cx) subunits consists of a gap junctional channel, called a connexon, and there are at least 21 Cx isoforms identified in vertebrates (Goodenough & Paul, 2009). Even though some types of Cx isoforms are widely present in various tissues, the expression of other Cx isoforms is relatively restricted in certain cells (Meşe et al., 2007). In addition, the co-expression of multiple Cx isoforms within a single cell is frequently observed, including testis and epididymis of the male reproductive tract (Meşe et al., 2007).
The excurrent duct of male reproductive tract includes the efferent ductules, epididymis, and vas deferens. The epididymis is the site in which spermatozoa released from the testis become mature and acquire fertilizing capacity (Robaire & Hermo, 1988). Histologically, the epididymis is a tubular structure having a lumen inside surrounded by a layer of epithelial cells (Robaire & Hermo, 1988). The epithelial cell layer is consisted of several types of cells, including principal, basal, narrow, and apical cells which have special morphological features and functions distinguishable from each other (Robaire & Hermo, 1988). In addition, the proportion of cell types present in the epididymal epithelial layer varies along the epididymal duct (Robaire & Hermo, 1988). Based on its structure and functions, the epididymis is divided into four different parts, initial segment, caput epididymis, corpus epididymis, and caudal epididymis (Robaire & Hermo, 1988). For example, the epithelium of caput epididymis is thicker than that of caudal epididymis (Robaire & Hermo, 1988). Also, the caput and corpus epididymis are histochemically specialized for luminal fluid absorption and secretion involved in sperm maturation, while the caudal epididymis is adapted for storage of sperm (Robaire & Hermo, 1988). Thus, it is generally considered that different compositions of epithelial cells would contri–bute to the regional difference of the epididymis, at least in part (Robaire et al., 2006).
The expression and presence of Cx isoforms in the epididymis during postnatal development have been examined by several previous researches (Cyr et al., 1996; Dufresne et al., 2003; Han & Lee, 2013; Lee, 2013). It is generally agreed that the expression of Cx isoforms in the epididymis follows the age-specific and segmental-specific manners (Dufresne et al., 2003; Han & Lee, 2013; Lee, 2013). For example, the highest expression of Cx26 in the caput epididymis is found at 5 months of age (Han & Lee, 2013), while the expression of Cx26 in the corpus epididymis becomes increased until 25 days of age, followed by a gradual decrease of Cx26 expression as aged (Lee, 2013). On the other hand, the expression of Cx26 in the caudal epididymis during postnatal development increases as aged and reaches the highest level at 2 years of age (Lee, 2013). However, even though expressional patterns of Cx isoforms in the epididymis are relatively well-documented, the cellular localization and expressional regulation of Cx isoforms in the epididymis have not been studied in detail. A significant decrease of Cx43 expression in the epididymis, except the caudal epididymal part, has been observed by propylthiouracil-induced hypothyroidism at the neonatal age (St-Pierre et al., 2003). In addition, our previous researches have shown that expression of Cx isoforms in the corpus and caudal epididymis at the adult is modulated by exogenous treatments of estradiol benzoate (EB), a potent estrogen agonist, or flutamide (Flu), an antiandrogenic compound, at the neonatal age (Lee, 2015; Lee, 2016). These observations suggest that expression of Cx isoforms in the epididymis is regulated by intrinsic and/or exogenous compounds in a complicate manner. Yet, the effect of EB or Flu treatment on the expression of Cx isoforms in the caput epididymis has not been tested. Thus, in the present study, the expressional changes of Cx isoforms in the adult caput epididymis after the neonatal exposure to EB or Flu were examined at the transcript level.
MATERIALS AND METHODS
Male pup rats were obtained from the delivery of pregnant Sprague Dawley rats acquired from Samtako (Osan, Korea). Once the arrival, each pregnant rat was individually separated and randomly designated to one of experimental groups, a control group (peanut oil), a low-dose estradiol benzoate treatment group [EB-L, 0.015 µg of EB/kg body weight (BW)], a high-dose EB treatment group (EB-H, 1.5 µg of EB/kg BW), a low-dose flutamide treatment group (Flu-L, 500 µg of Flu/kg BW), or a high-dose Flu treatment group (Flu-H, 5 mg of Flu/kg BW). Free access to drinking water and food for animals were permitted for whole experimental period.
A subcutaneous injection of peanut oil for control group or pre-made EB or Flu solution for treatment groups was performed to an experimental animal at 1 week of age. The EB or Flu powder purchased form Tokyo Chemical Industry Co. (Tokyo, Japan) was dissolved in 100% EtOH, and the EB or Flu solution was then diluted in peanut oil to make working solution. An adequate amount of EB or Flu solution to be administrated was calculated from body weight measured at 1 week of age. The maximum volume of the injected solution per animal was not over 0.05 mL. The number of animals utilized for the present study is as following; control group (n=6), EB-L group (n=6), EB-H group (n=6), Flu-L group (n=6), and Flu-H group (n=6). The present study was carried out in accordance with the guide for the care and use of laboratory animals of National Research Council in S. Korea.
Once the animal became 4 months of postnatal age, anesthetization by CO2 stunning was performed. The male reproductive tract was taken out from an incision on lower abdomen and was placed on the cold PBS solution. The epi–didymis was first separated from the testis and other reproductive tract and was further dissected into different epidi–dymal segments, including caput epididymis. The tissue was briefly washed in fresh cold PBS solution once and was quickly frozen in liquid nitrogen. The tissue was kept in –80℃ until utilized for total RNA isolation.
To extract total RNA from the caput epididymis, the frozen tissue was completely homogenized in total RNA extraction solution (iNtRON Biotech, Sungnam, Korea). Total RNA was precipitated by isopropanol, and total RNA pellet was washed with 70% DEPC-EtOH. Then, air-dried total RNA was dissolved in DEPC- dH2O. The concentration of total RNA was assessed with NanoDrop Lite spectrophotomer (Thermo Scientific, Wilmington, DE). The quality of total RNA was evaluated by conventional agarose gel electrophoresis.
The first-stranded cDNA was generated with 1 µg of total RNA, oligo-dT primer, and ImProm-IITM reverse transcription system (Promega, Madision, WI) in a total volume of 20 µL. The reverse transcription reaction was carried out in a serial process of 25℃ for 5 min, 42℃ for 90 min, and 70℃ for 15 min. The cDNA construction was used for quantitative real-time PCR analysis as below. Oligonucleotide primers utilized for quantitative real-time PCR analysis are listed in Table 1.
One microliter of cDNA, in addition to 10 pmol primer set, 10 µL PCR master mixture (Finnzymes, Espoo, Finland), and DNase-free dH2O, was used to conduct the quantitative real-time PCR. The mixture of PCR in a total volume of 20 µL was placed in a thermocycler (Bio-Rad Laboratories, Hercules, CA) and was undergone a sequential procedure as followings; a pre-denaturation step at 95℃ for 30 sec, 40 cycles of denaturation at 95℃ for 30 sec, annealing at Tm for 30 sec, and extension at 72℃ for 30 sec, and an additional extension step at 72℃ for 5 min. For a quality control purpose of PCR, cyclophilin A (Ppia) was included in every reaction.
For each experimental group, independently quadruplicated RT reactions and PCRs were performed to obtain a mean and a standard error. The experimental data were presented in the relative expressional ratio between Ppia and Cx isoform. Statistical significances of Cx expression between control and EB or Flu treatment groups were evaluated by one-way ANOVA, followed by Duncan’s test. If P is less than 0.05, it was regarded as the existence of statistical difference among experimental groups.
RESULTS
The treatment of EB at 1 week of age didn’t influence on gene expression of Cx26 in the adult caput epididymis (Fig. 1A). However, a significant increase of Cx26 transcript level was observed with a low-dose Flu treatment, even though expression of Cx26 was not significantly changed by a high-dose Flu treatment (Fig. 1A).
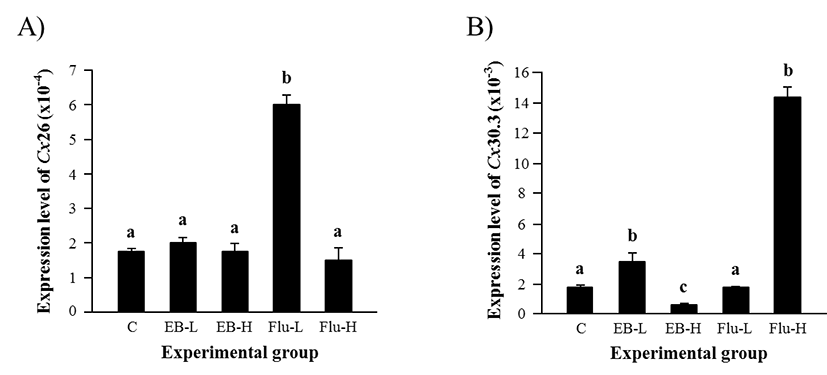
Expression of Cx30.3 was significantly increased by a low-dose EB treatment, while a reduction of Cx30.3 transcript level was detected by a high-dose EB treatment (Fig. 1B). The treatment of a low-dose Flu at 1 week of age didn’t affect the expression of Cx30.3 in the adult caput epididymis (Fig. 1B). However, a tremendous increase of Cx30.3 transcript amount in the caput epididymis at the adult was observed with a high-dose Flu treatment at 1 week of age (Fig. 1B).
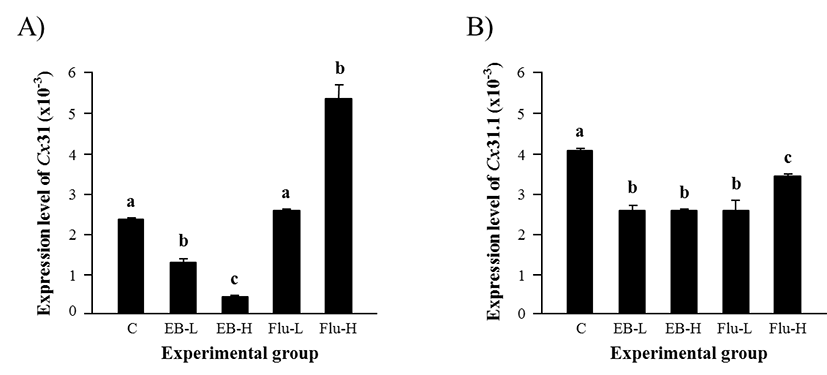
Expression of Cx31 in the adult caput epididymis was significantly decreased by a low-dose EB treatment (Fig. 2A). An additional reduction of Cx31 transcript level was observed in a high-dose EB-treated group, as shown in Fig. 2A. There was no change of Cx31 transcript amount by a lowdose Flu treatment, but a significant increase of Cx31 expression was detected in a high-dose Flu-treated group (Fig. 2A). The expression of Cx31.1 isoform in the adult caput epididymis was declined in all treatment groups (Fig. 2B). The EB treatment resulted in a significant decrease of Cx31.1 transcript level, even though there was no significant difference on Cx31.1 expression between two doses (Fig. 2B). The Flu treatment also caused significant reduction of Cx31.1 transcript level, but the effect of Flu treatment on Cx31.1 expression was more severe with a low-dose treatment than a high-dose treatment (Fig. 2B).
The expression of Cx32 in the adult caput epididymis after the exposure to EB at 1 week of age was significantly decreased by a low-dose treatment, but not by a high-dose treatment (Fig. 3A). On the other hand, the treatment of a low-dose Flu didn’t affect the expression of Cx32, but a significant decline of Cx32 transcript level was detected with a high-dose Flu treatment (Fig. 3A).
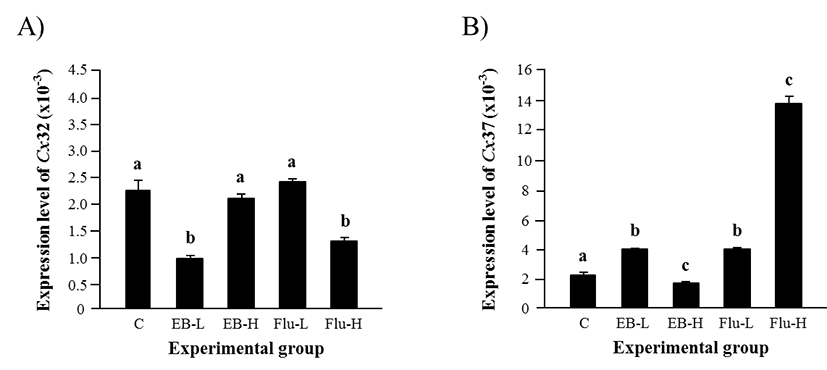
The effect of EB or Flu treatment at 1 week of age on the expression of Cx37 in the adult caput epididymis is shown in Fig. 3B. The treatment of a low-dose EB resulted in a significant increase of Cx37 expression, but a high-dose EB treatment caused a decrease of Cx37 transcript level (Fig. 3B). Exposure to a low-dose Flu at 1 week of age led into a major increase of Cx37 expression in the adult caput epididymis, and a further rise of Cx37 transcript level was induced by a high-dose Flu treatment (Fig. 3B).
Expression of Cx40 in the adult caput epididymis was significantly down-regulated by the treatment of EB at 1 week of age, even though the level of Cx40 transcript was not statistically different between a low-dose and a high-dose EB treatment (Fig. 4A). However, the Flu treatment at a low-dose resulted in a significant increase of Cx40 transcript levels (Fig. 4A). The treatment of a high-dose Flu induced an additional surge of Cx40 expressional level, as shown in Fig 4A.
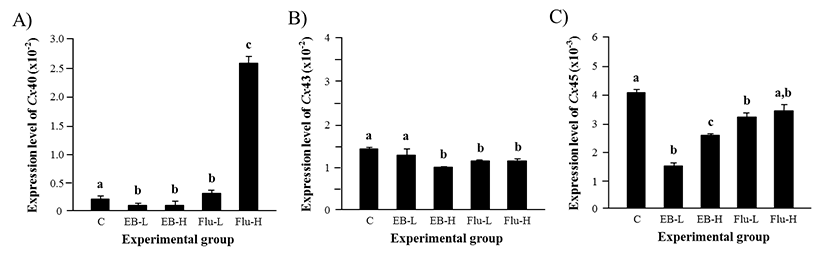
Expression of Cx43 in the caput epididymis was not affected by a low-dose EB treatment (Fig. 4B). But, a significant decrease of Cx43 transcript level was observed with a high-dose EB treatment (Fig. 4B). The treatment of Flu caused the reduction of Cx43 transcript level, although there was no significant difference on the expression of Cx43 between the treatments at a low-dose and a high-dose Flu (Fig. 4B).
The transcript level of Cx45 was significantly decreased by a low-dose EB treatment, as seen in Fig. 4C. The treatment of a high-dose EB also caused the reduction of Cx45 transcript level (Fig. 4C). However, the abundance of Cx45 transcript in a high-dose EB treatment group was higher than that in a low-dose EB treatment group (Fig. 4C). A low-dose Flu treatment led into a significant decrease of Cx45 expression in the adult caput epididymis, even though a high-dose Flu treatment didn’t cause a change of Cx45 transcript level (Fig. 4C).
DISCUSSION
Expressional alternation of Cx isoforms in the adult corpus and caudal epididymis by EB or Flu treatment at neonatal age has been previously examined (Lee, 2015; Lee, 2016). The current research has investigated the effect of EB or Flu treatment at 1 week of postnatal age on the expression of Cx isoforms in the caput epididymis at the adult. The findings are summarized as follows: (1) the exposure to EB at a low dose results in significant decreases of the expression of most Cx isoforms in the caput epididymis, except Cx30.3 and Cx37 which the expression has become increased and Cx26 and Cx43 with no significant expressional changes: (2) the treatment of EB at a high dose at 1 week of postnatal age generally causes significant decreases of most Cx isoforms in the caput epididymis at the adult, but not Cx26 and Cx32 showing no significant change of the expressional level: (3) the effect of a low-dose Flu treatment leads to increases of Cx37 and Cx40 expression, decreases of Cx31.1, Cx43, and Cx45 expression, and no expressional change of Cx26, Cx30.3, Cx31, and Cx32: and (4) the administration of a high-dose Flu at 1 week of age results in significant expressional increases of Cx30.3, Cx31, Cx37, and Cx40 and significant expressional decreases of Cx31.1, Cx32, and Cx43 in the adult caput epididymis.
It is well known that the epididymis is a representative androgen-responsive tissue in the male reproductive tract (Robaire & Hinton, 2006). Testosterone and dihydrotestosterone play important roles on the proper differentiation and maturation of the epididymis (Robaire & Hinton, 2006). However, Atanassova et al (2001) have demonstrated that expression of estrogen receptor α (ERα) in the epididymis is segmental-specific and postnatal age-specific. Also, others have shown the aberrant gene expression in the epididymis of the adult after the exposure to EB during neonatal period (Putz et al., 2001). Moreover, Gorowska et al (2014) have found aberrant gene expression in the epididymis exogenously treated with steroidal and/or anti-steroidal compounds. Thus, these findings suggest that the epididymis could be a target tissue of not only androgen but also estrogen. Indeed, our previous researches have also shown abnormal expression of Cx isoforms in the adult corpus and caudal epididymis exposed with EB or Flu at the neonatal age (Lee, 2015; Lee, 2016). However, it seems that expressional regulation of Cx isoforms in different epididymal parts does not fit into a uniformed manner. For instance, with a low-dose EB treatment at 1 week of age, there is no expressional change of Cx43 in the caput epididymis of the adult. But, in the same circumstance, expression of Cx43 is greatly increased in the corpus epididymis, but not in caudal epididymis (Lee, 2015; Lee, 2016). In addition, even though the treatment of a low-dose Flu at 1 week of age causes a significant increase of Cx32 expression in the adult caput epididymis, the same treatment does not give an influence on the expression of Cx32 in adult corpus and caudal epididymis (Lee, 2015; Lee, 2016). Due to the complexity of data obtained, it is hard to conclude the role of estrogen and/or androgen on the expression of each Cx isoform in the epididymis at this point. But, it seems to be clear that exogenous exposure to EB or Flu at the early postnatal age affects the expression of Cx isoforms in the entire epididymal segments. Also, it is likely that the expressional regulation of Cx isoform by androgenic and/or estrogenic substances is segmental-specific in the epididymis. Like promoter assay and gene expression analysis, detailed molecular approaches would provide valuable information to explain differential expressional regulation of each Cx isoform at the different epididymal parts.
The expression of androgen receptor (AR) and ER in the epididymis during postnatal development is fluctuated and shows segmental-specific patterns (Atanassova et al., 2001; You & Sar, 1998). Additionally, until 14 days of postnatal age, the differentiation of epithelial cells in the epididymis does not begin (Robaire et al., 2006). At the 3th week of postnatal age, columnar cells are derived from undifferentiated epithelial cells, and the columnar cell becomes either the basal or principal cell around 4th week of postnatal age (Robaire et al., 2006). The appearance of other cell types, including narrow and clear cells, start after 35 days of postnatal age (Robaire et al., 2006). Because the postnatal maturation of the epididymis is dependent on androgenic and/or other various hormonal factors, any elements affecting hormonal homeostasis during the early postnatal period could give an influence on the differentiation of the epithelial cell in the epididymis and furthermore numbers of epithelial cell types in the adult epididymis. Cyr et al (1996) have demonstrated the limited localization of Cx43 between basal and principal cells, but not between adjacent principal cells. Thus, if EB or Flu treatment at 1 week of postnatal age influences on the differentiation of the epithelial cells in the epididymis, it is supposed that the proportion of epithelial cell types in the caput epididymis at the adult would be changed and thus the expression of Cx isoforms, including Cx43, would be altered.
In conclusion, based on previous and present findings, the expression of Cx isoforms in the epididymis is differentially regulated in segment-specific manner. Even though a precise regulatory mechanism of expression of Cx isoforms in the epididymis has not been provided, it is evident that the exposure to estrogenic and/or anti-androgenic substance during the early postnatal development could impact on the expression of normal expression of Cx isoforms in the adult epididymis and presumably epididymal functions at the adult.

