INTRODUCTION
The functional accordance of complex tissues in the male reproductive tract is required for successful male fertility. The male reproductive tract is generally consisted of the testis and excurrent ducts, and the excurrent ducts are further divided into the efferent ductules, epididymis, and vas deferens (Robaire & Hermo, 1988). Even though spermatozoa are produced from the testis, proper maturation of the spermatozoa occurs in the epididymis and is also crucial to maintain male reproduction (Robaire & Hermo, 1988). In this viewpoint, it is no doubt that the development of fully functional epididymis is essential for production of mature sperms.
Based on morphological and functional aspects, the epididymis has four discrete regions, including initial segment, caput epididymis, corpus epididymis, and cauda epididymis (Robaire & Hermo, 1988). The epididymis is consisted of a single layer of epithelial cell surrounded by smooth muscle layers (Robaire & Hermo, 1988). The epididymal epithelium has several cell types, including principal, basal, halo, narrow, and apical cells (Arrotéia et al., 2012; Robaire & Hermo, 1988). The composition of cell type in the epithelium varies along the epididymal duct, i.e. principal cell is predominantly localized in the caput epididymis, while narrow cell is more frequently found in cauda epididymis than caput epididymis (Arrotéia et al., 2012). In the adult epididymis, each cell type is characterized with specific structural and histological features, and it is generally believed that different cell type plays various specific roles, eventually contributing to epididymal functions (Robaire & Hermo, 1988). Thus, different composition of cell types along the epididymal duct seems to influence on regionalization of specialized epididymal functions.
Differentiated epithelial cell types in the epididymis are not seen in the early neonatal age. Differentiation of epididymal cells in the rat epididymis begins at 14 days of postnatal age and is completed around 50 days of postnatal age (Rodriguez et al., 2002). The halo cells appear at postnatal day 14, and the early differentiation of primitive undifferentiated cells to the narrow and columnar cells is approximately observed at postnatal day 21 (Rodriguez et al., 2002). The columnar cells differentiate into basal and principal cells at around day 28, and full differentiation of clear cells at around 50 days of postnatal age indicates the completion of differentiation of the epididymal epithelium (Rodriguez et al., 2002). Such sequential differentiation of epithelial cell types in the epididymis in age-dependent manner suggests that exposure to exogenous elements during the early postnatal development could influence on differentiation and normal development of the epididymal epithelial cells.
Cell-cell communication is particularly important for the maintenance of homeostasis of a given tissue or organ, especially composed of several cell types, such as the epididymis. Of ways of cell-cell interaction, direct cellular communication via gap junction is essential for synchronization, differentiation, cellular growth, and metabolic coordination of a specific tissue (Meşe et al., 2007). Gap junctions are intercellular channels linking the cytoplasm of neighboring cells and allow for exchange of ions, small metabolites, second messengers, and even RNAs between cells (Lawrence et al., 1978; Valiunas et al., 2005). Each gap junction is composed of 6 connexins (Cxs), and there are 21 Cx isoforms detected in vertebrates (Goodenough and Paul, 2009). Even though almost all mammalian cells possess a type of Cx isoforms, expression of Cx isoforms is dependent on cell types during development (Goodenough and Paul, 2009). Especially, in the male reproductive tract, differential expression of Cx isoforms in the testis and epididymis during postnatal period has been identified from our earlier researches and others (Cry, 2011; Lee, 2013; Seo et al., 2010). Our other previous findings demonstrates that expression of Cx isoforms in the initial segment of the adult rat epididymis is altered by exogenous exposure to estrogen agonistic or testosterone antagonistic compound at early postnatal ages (Lee, 2014, 2015a). In addition, another previous study shows that administration of estrogen agonistic or testosterone antagonistic compounds at 7 days of postnatal age results in modulation of expression of several Cx isoforms in the adult corpus epididymis (Lee, 2015b). Together, these researches exhibit that expression of Cx isoforms in the epididymis could be influenced by steroid hormones during postnatal developmental time. Moreover, these previous findings suggest that different expressional patterns of Cx isoforms could be induced by doses and postnatal age exposed to the steroid hormone agonist or antagonist.
Based on the previous findings, it was speculated that treatment of estradiol benzoate (EB) or flutamide (Flu) at later than 7 days of postnatal age would result in different expressional patterns of Cx isoforms in the adult corpus epididymis. Thus, the present research was designed to determine the effects of exposure to EB or Flu at the weaning age on expression of Cx isoforms in the adult cauda epididymis.
MATERIALS AND METHODS
A total of five pregnant Spragure Dawley female rats were obtained from Samtako (OSan, Korea) and separately kept during the entire experimental period. Each female rat was randomly assigned into one of 5 experimental groups, including a control (vehicle-treated), two estradiol benzoate (EB)-treated, and two flutamide (Flu)-treated groups. EB or Flu-treated groups were further divided into a low-dose EB-treated (EB-L, 0.015 μgof EB /kg body weight (BW)) and a high-dose EB-treated (EB-H, 1.5 μgof EB /kg BW) or a low-dose Flu-treated (Flu-L, 500 μgof Flu/kg BW) and a high-dose Flu-treated (Flu-H, 5 mg of Flu/kg BW) group. Free access to food and drinking water was allowed. Upon the delivery, 5 to 7 male pups were obtained from each female rat.
The solution of EB and Flu for subcutaneous injection was prepared as following. The EB and Flu powder was purchased from Tokyo Chemical Industry Co. (Tokyo, Japan), and dissolved in 100% EtOH and then diluted in peanut oil. At the weaning age, 21 days of postnatal age, body weight of each pup was measured, and a single shot of diluted EB or Flu solution at the maximum volume of 0.05 mL was injected. The control group was administrated with same amount of peanut oil. A total of 31 male pups were used for the present research, including control (n=5), EB-L (n=7), EB-H (n=7), Flu-L (n=6), and Flu-H (n=6) groups.
The experimental animals were anesthetized by CO2 stunning at 4 months of postnatal age. The male reproductive tract was exposed through an incision on lower abdomen, and the epididymis was separated from the testis and vas deferens in cold PBS. The epididymis was rapidly dissected into initial segment, caput epididymis, corpus epididymis, and cauda epididymis. Each part of the epididymis washed in fresh cold PBS twice was frozen in liquid nitrogen and stored in –80℃ until utilized for total RNA isolation later.
A process based on phenol-chloroform extraction method was employed to isolate total RNA from the corpus epididymis. Briefly, the tissue was homogenized in total RNA extraction solution (iNtRON Biotech, Sungnam, Korea) with a homogenizer (Fisher Scientific, Pittsburgh, USA). Total RNA was precipitated with isopropanol, and a pellet of total RNA was washed in 70% EtOH and dissolved in DEPC-treated RNase-free dH2O. Concentration and purity of total RNA isolated were evaluated with NanoDrop Lite spectrophotometer (Thermo Scientific, Wilmington, USA), and quality of total RNA was determined by gel electrophoresis. Total RNA samples were directly used for construction of cDNAs.
One microgram of total RNA was used to generate cDNA using oligo-dT primer and ImProm-IITM reverse transcription system (Promega, Madison, USA), and the reverse transcription reaction was achieved with a sequential process of 25℃ for 5 min, 42℃ for 90 min, and 70℃ for 15 min. The cDNAs were independently prepared with total RNAs isolated from three animals per experimental group. The generated cDNA was directly used for a template of quantitative realtime PCR. Information of oligonucleotide primers used for real-time PCR analysis is shown in Table 1.
A mixture of 1 μL of cDNA, 10pmol of primer set, 10 μL of PCR master mixture (Finnzymes, Espoo, Finland), and DNase-free dH2O to make a final volume of 20 μL was prepared as the PCR cocktail. The PCR was performed as followings; a pre-denaturation at 95℃ for 30 sec, cycles of denaturation at 95℃ for 30 sec, annealing at Tm for 30 sec, and extension at 72℃ for 30 sec. The final extension at 72℃ for 10 min was included at the end of each PCR. For quality control purpose, cyclophilin A (Ppia) was used for a PCR internal control. The PCR products were fractionated in 1.2% agarose gel to confirm the sizes of the products.
The reverse transcription reaction and PCR were independently triplicated to obtain a mean and a standard error of an experimental group. The experimental data are present in the relative expressional ratio between Ppia and target Cx isoform. Statistical significances among experimental groups of EB or Flu treatment, compared with control, were determined by one-way ANOVA, followed by Duncan’s test if necessary. When P value was lower than 0.05, it was considered the presence of a statistical significance between experimental groups.
RESULTS
There was no significant effect of Cx26 expression by a low-dose EB treatment, while about one fold increase of Cx26 transcript level was detected with a high-dose EB treatment (Fig. 1A). Expression of Cx26 in adult corpus epididymis was changed by a low-dose Flu treatment, but a high-dose Flu treatment resulted in a significant decrease of Cx26 expression in the corpus epididymis of adult rat (Fig. 1A).
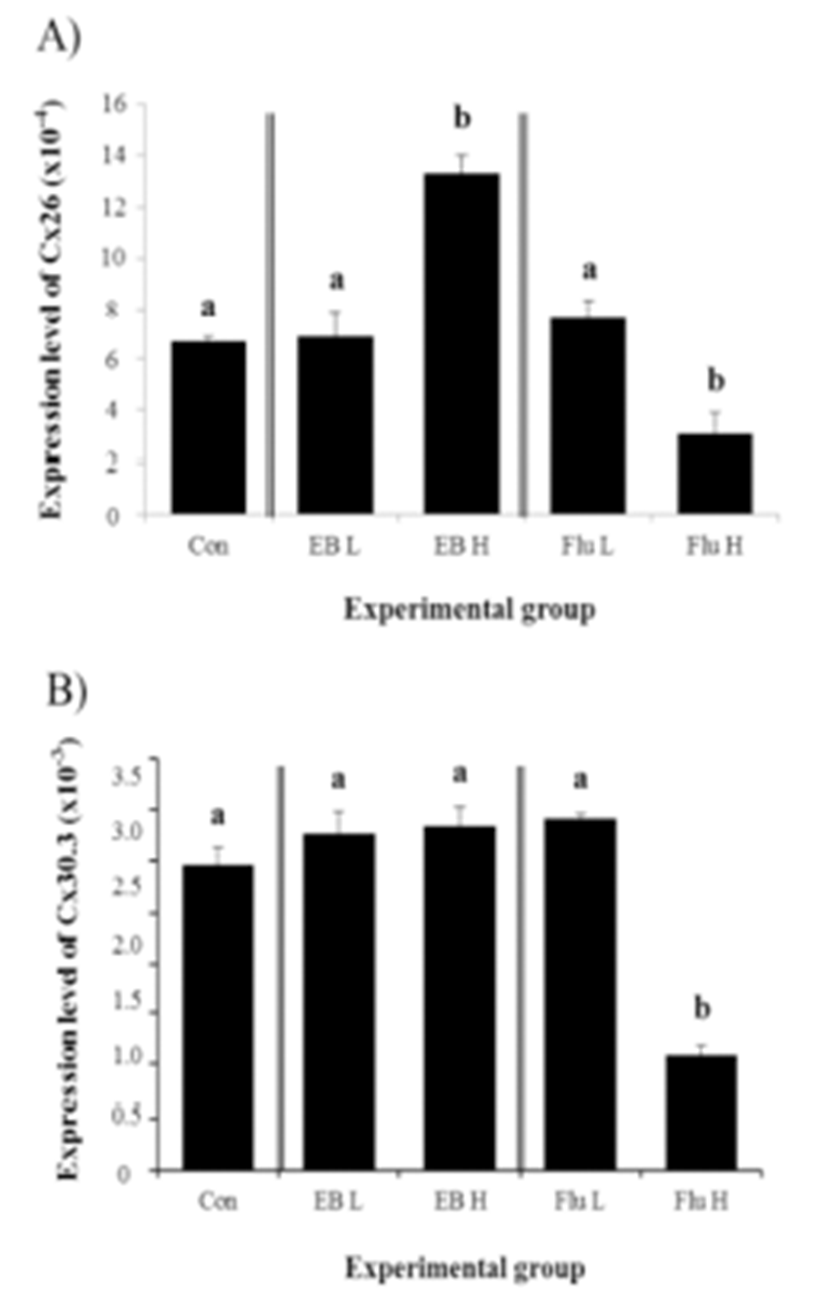
Expression of Cx30.3 in the adult corpus epididymis after the exposure to EB or Flu at the weaning age was not significantly changed, except a high-dose Flu treatment causing a drastic reduction of Cx30.3 expression, as shown in Fig. 1B.
The treatments of EB at the weaning age, regardless of the dose, caused significant increases of Cx31 gene expression in the corpus epididymis at 4 months of age (Fig. 2A). The high-dose EB treatment didn’t result in a significant change on Cx31 transcript level, compared with that with low-dose ED treatment (Fig. 2A). However, a treatment with high-dose of Flu led into a significant reduction of Cx31 transcript level, while there was no change of Cx31 expression with a low-dose Flu treatment (Fig. 2A).
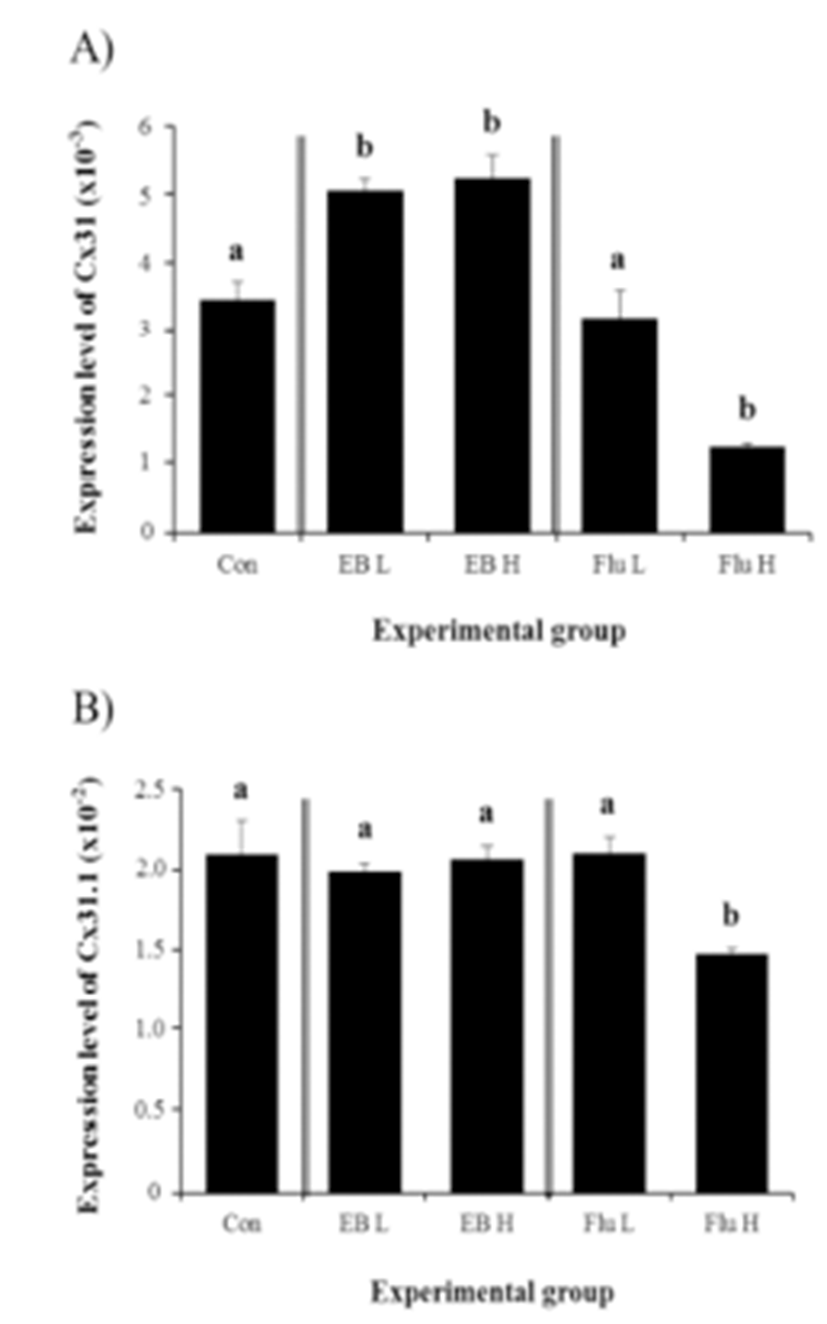
The exposure to EB at the weaning age didn’t influence on the expression of Cx31.1 in the corpus epididymis at the adult (Fig. 2B). However, a high-dose Flu treatment at the weaning age resulted in a significant decrease of Cx31.1 expression in the adult corpus epididymis, even though there was no statistical change on Cx31.1 transcript level by a low-dose Flu treatment (Fig. 2B).
Expressional changes of Cx32 gene were detected with the EB treatment (Fig. 3A). Regardless of the dose of the treatment, the exposure to EB at the weaning age resulted in a significant increase of Cx32 transcript level in the corpus epididymis at the adult (Fig. 3A). However, there was no difference on expressional level of Cx32 by the dose of EB treated (Fig. 3A).
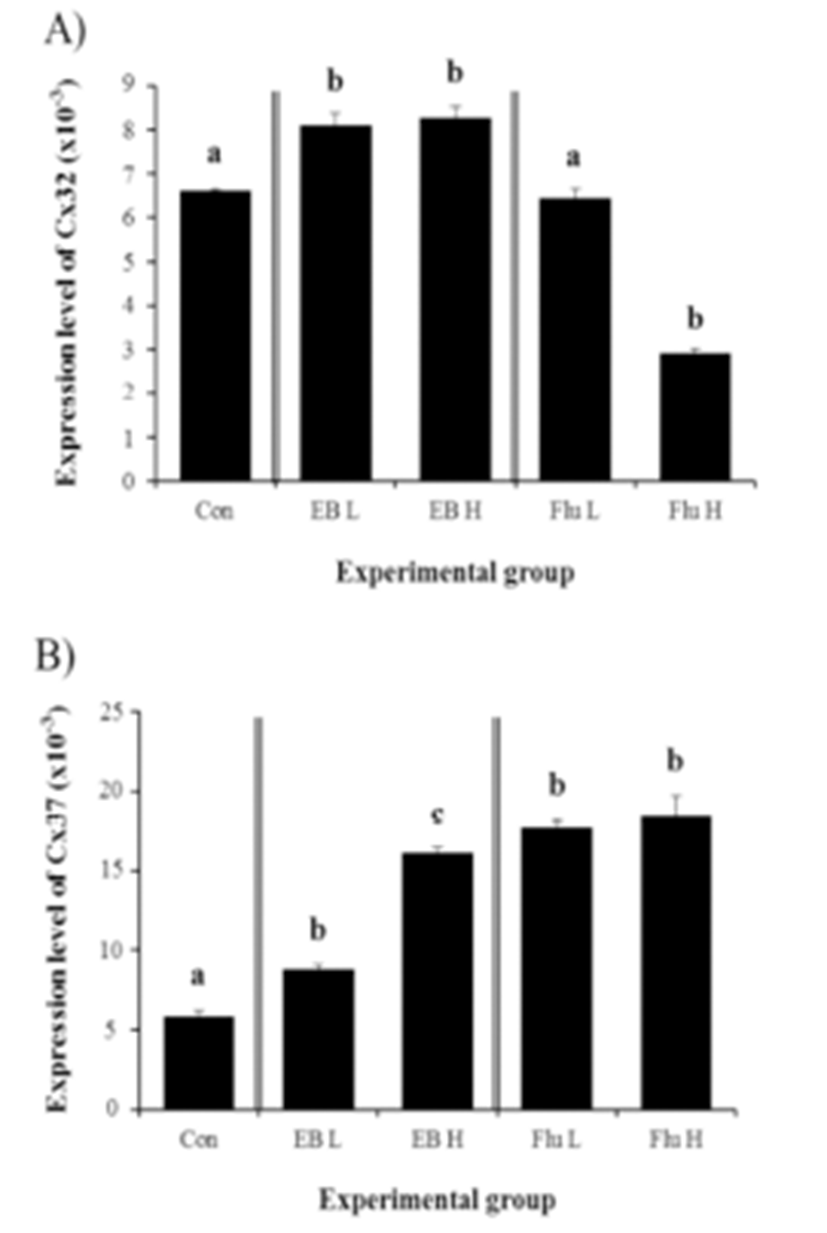
The treatment of a low-dose EB at the weaning age caused a significant increase of Cx37 transcript level (Fig. 3B). A further increase of Cx37 mRNA abundance in the adult corpus epididymis was observed with a high-dose EB treatment (Fig. 3B). Even though there was no significant difference on expressional level of Cx37 between treatments at two different doses of Flu, the exposure to Flu at the weaning age resulted in a significant increase of Cx37 transcript level in the adult corpus epididymis (Fig. 3B).
The expression of Cx40 in the adult corpus epididymis was significantly increased by the exposure to EB at the weaning age (Fig. 4A). However, there was no difference on Cx40 expression by the dose of EB treated (Fig. 4A). Unlike the EB, the treatment of Flu at the weaning age didn’t influence on expression of Cx40 in the adult corpus epididymis (Fig. 4A).
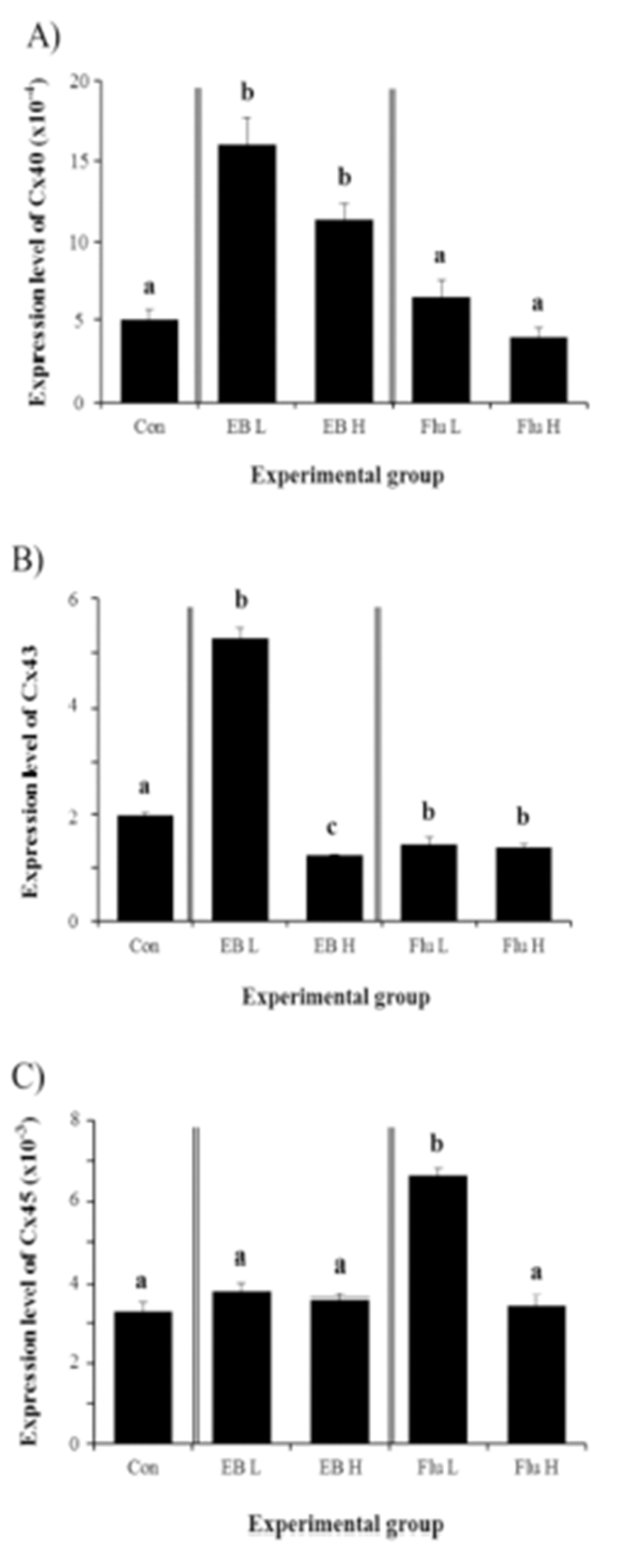
The treatment of a low-dose EB resulted in a significant increase of Cx43 expression in the corpus epididymis at the adult (Fig. 4B). However, a significant decrease of Cx43 transcript level was found in a treatment of high-dose EB (Fig. 4B). Treatments of Flu at two different doses caused significant decreases of Cx43 gene expression, even though there was no difference on level of Cx43 transcript between two treatments (Fig. 4B).
Expression of Cx45 in the corpus epididymis at the adult was not changed by EB treatment at the weaning age (Fig. 4C). A significant increase of Cx45 transcript level was detected in a low-dose Flu treatment, even though treatment of a high-dose Flu didn’t give an influence on Cx45 mRNA abundance (Fig. 4C).
DISCUSSION
Disruption of endogenous levels of steroid hormones by exogenous exposure to steroidal substances during the early postnatal period frequently results in abnormal gene expression in steroid hormone-sensitive tissue or organ in adult male, such as the epididymis and prostate (Gorowska et al., 2014). Expressional changes of Cx isoforms in the initial segment of adult rat after treatments with EB or Flu at neonatal and weaning ages have been observed from our previous researches (Lee, 2014; 2015a). Recently, aberrant gene expression of Cx isoforms has been detected in adult corpus epididymis exposured to EB or Flu at neonatal age (Lee, 2015b). The current study showed that EB or Flu treatment at weaning age also caused disruption of normal expression of Cx isoforms in the adult corpus epididymis.
Expressional regulation of multiple Cx isoforms by steroid hormones in the corpus epididymis has been suggested from our previous research (Lee, 2015b). Significant decreases on Cx26 and Cx30.3 transcript levels in adult corpus epididymis are observed with treatment of EB at the highdose or Flu at the low- and high-doses at 7 days of postnatal age (Lee, 2015b). However, in the present study, significant decreases of Cx26 and Cx30.3 mRNA levels were only detected with the treatment of a high-dose Flu at 21 days of postnatal age. Exposure to a high-dose EB rather led into a significant increase of Cx26 transcript level. From findings of our previous and current researches, it is resolved that the low-dose of EB is not powerful enough to give an impact on expression of Cx26 and Cx30.3 genes but the high-dose of Flu effectively downregulates expression of Cx26 and Cx30.3 genes. Compared with our previous research (Lee, 2015b), treatment of the high-dose of EB or low-dose of Flu at different postnatal age results in different outcomes in expression of Cx26 and Cx30.3 genes. These observations indicate that expression of Cx26 and Cx30.3 gene is differentially regulated by estrogenic and/or androgenic substance, depending on postnatal ages. It is not certain at this point that such differential responsiveness to EB or Flu on expression of Cx26 and Cx30.3 gene is due to different level of estrogen receptor or androgen receptor in the corpus epididymis at different postnatal age and/or different level of steroid hormone secreted from the developing testis. Examination of expressional changes of these receptors in the corpus epididymis during the early postnatal development would provide information to resolve such differential effect of EB or Flu on gene expression of Cx26 and Cx30.3.
Treatment of EB or Flu at different postnatal age results in differential gene expression of other Cxs in the corpus epididymis. Expression of Cx31 and Cx31.1 is significantly decreased in adult corpus epididymis by the high-dose EB or both-doses Flu at 7 days of postnatal age (Lee, 2015b). However, treatments of both-doses EB at 21 days of postnatal age cause increases of Cx31 gene expression, while there is no significant change on expression of Cx31 gene by the low-dose Flu treatment at 21 days of postnatal age. In addition, expression of Cx31.1 gene is not affected by EB treatment and is only influenced by treatment of the highdose Flu at 21 days of age. It is possible that such differential expression of Cx31 and Cx31.1 genes to EB or Flu treatment could be due to different sensitivity to estrogenic or androgenic compound at different postnatal age. In addition, we can not rule out the existence of different regulatory mechanisms for Cx31 and Cx31.1 expression by EB or Flu at different postnatal age.
Expressional increases of Cx32 and Cx37 genes after the low-dose EB treatment are also observed from our previous study (Lee, 2015b). However, expression of Cx32 is significantly decreased with the high-dose EB treatment at 7 days of postnatal age (Lee, 2015b), while the same treatment at 21 days of age results in an increase of Cx32 gene expression in adult corpus epididymis. Differential expressional patterns of Cx32 and Cx37 genes are more apparent with Flu treatment at different postnatal age. Expression of Cx32 is decreased by treatments of two doses of Flu at 7 days of age (Lee, 2015b), but exposure to the high-dose Flu, not the low-dose Flu, at 21 days of postnatal age only causes a decrease of Cx32 gene expression. Moreover, treatments of two-doses Flu at 21 days of postnatal age increase expression of Cx37 gene, while a decrease or no change of Cx37 mRNA level is detected with the treatments of the low-dose Flu or high-dose Flu at 7 days of age, respectively (Lee, 2015b). At the current point, it is very hard to interpret the effects of EB or Flu treatment at different postnatal age on expression of Cx32 and Cx37 in adult corpus epididymis. Clearly, these findings propose that expression of Cx32 and Cx37 in the corpus epididymis is under the regulation of estrogenic and/or androgenic compound. In addition, it is strongly suggested that expressional regulation of Cx32 and Cx37 during postnatal period is far complicate. In future, detailed molecular examinations are recommended to resolve the actions of steroid hormones on regulation of Cx32 and Cx37 gene expression.
Treatment of EB or Flu at different postnatal age induces quite different patterns of expression of Cx40, Cx43, and Cx45 in adult corpus epididymis. Expression of Cx40 is significantly decreased by the high-dose EB or Flu administration at 7 days of postnatal age (Lee, 2015b), while two doses of EB treatments at 21 days of postnatal age result in increases of Cx40 expression and Flu treatment doesn't influence on expression of Cx40 gene. In case of Cx43, exposure to the high-dose of EB and low-dose of Flu at 7 days of age causes reduction of the transcript levels in adult corpus epididymis (Lee, 2015b). However, except the low-dose EB treatment leading into an increase of Cx43 gene expression, other treatments at 21 days of age result in significant decreases of Cx43 expression. Expressional patterns of Cx45 gene in adult corpus epididymis by exposure to EB or Flu depend on postnatal age of the treatment. When the EB or Flu is administrated at 7 days of postnatal age, the low-dose EB treatment causes an increase of Cx45 expression but other treatments result in significant expressional decreases of Cx45 gene (Lee, 2015b). A change of Cx45 expression by the treatment of EB or Flu at 21 days of postnatal age is only detected with the low-dose Flu treatment. Of several possible suggestions to explain such inconsistency on expression of Cx isoforms in adult corpus epididymis to the treatment of EB or Flu at different postnatal ages, age-dependent change of androgen receptor (AR) and/or estrogen receptor (ER) concentration in the corpus epididymis during postnatal period would be suitable (Cooke et al., 1991; Atanassova et al., 2001). Because the epididymis is an androgen- and estrogen-responsive tissue (Hess et al., 2011; Robaire and Hamzeh 2011) and concentrations to androgen and testicular estrogen vary during postnatal period (Hess 2003; Lee et al., 1975), disruption of endogenous concentrations of these steroid hormones by EB or Flu treatment at different postnatal age could bring on differential regulation on expression of various epididymal genes, including Cx isoforms.
In conclusion, the current research shows that expression of Cx isoforms in adult corpus epididymis could be modulated by exogenous exposure to estrogenic and/or androgenic substance at the weaning age. Data achieved from the present study do not directly demonstrate functional changes of the corpus epididymis due to abnormal expression of Cx isoforms by EB or Flu treatment at the weaning age. However, because cell-cell communication via gap junction is chiefly important for functional accordance in a tissue having various cell types, such as the corpus epididymis, it is generally reasonable to consider that disruption of normal expression of Cx isoforms by EB or Flu treatment at the weaning age could affect the epididymal functions.

