INTRODUCTION
Functional and structural maturation of male reproductive tissues are mostly dependent on the actions of androgens, as well as estrogens (Akingbemi & Hardy, 2001; Carreau et al., 2008). The epididymis is a part of excurrent ducts in male reproductive tract and is divided into four regions, including initial segments (IS), caput epididymis, corpus epididymis, and cauda epididymis, depending on morphological and functional features (Cosentino & Cockett, 1986). The epithelium of IS is characterized with the presence of narrow and apical cells which do not exist throughout the rest of the epididymis (Robaire & Hermo, 1988). An importance of the IS for proper sperm maturation has been examined by Temple-Smith et al. (1998). Surgical redirection of spermatozoa produced from the testis to the distal part of the caput epididymis results in significant reduction of the fertility (Temple-Smith et al., 1998). In addition, the luminal composition and epithelial enzymatic distribution within the IS are distinguished from the rest of the epididymis (Adamali & Hermo, 1996; Jones, 1987). Based on these findings, it is considered that the IS plays an important role discriminated from the other parts of the epididymis on sperm maturation.
Direct cell-cell communication is a way for regulation and maintenance of functions of tissues and/or organs in coordinated manner. There are three types of direct cell interaction between neighboring cells, including cadherin-based adhesion, occluding-based attachment, and connexin (Cx)-based gap junction (Pointies et al., 2005). Of these ways, cell-cell communication by gap junction allows direct exchange of signal molecules, RNAs, and ions through the cytoplasm of neighboring cells (Goodenough et al., 1996). Of 20 Cx isoforms identified in mammals, some Cx isoforms are present in virtually all cell types and others are expressed in strict cell types (Meşe et al., 2007). The presence and expression of Cx isoforms in the male reproductive tract are exhibited by others and our previous findings. Focused on the epididymis, Dufresne et al. (2003) demonstrate that expression of some Cx isoforms during postnatal development is segmentally specific along the epididymis. Our earlier researches have revealed different expressional levels of Cx isoforms in different epididymal regions at various postnatal ages (Han & Lee, 2013; Lee, 2013). In addition, our previous research shows differential expression of Cx isoforms in the IS from 1 week to 2 years of postnatal age (Seo et al., 2010). These results imply that postnatal maturation and functional regulation of the IS would relate with exact expression of Cx isoforms at correct ages. However, expressional regulation of Cx isoforms in the IS is caught less attention. Induced hypothyroidism at neonatal age causes a significant expressional decrease of Cx43 gene in the adult IS (St-Pierre et al., 2003). Also, it seems that epidermal growth factor involves in regulation of Cx43 expression in human cauda epididymis (Dubé et al., 2012). Moreover, our recent research demonstrates disruption of expression of Cx isoforms in the adult IS by exposure to estradiol benzoate (EB), an estrogen agonist, and/or flutamide (Flu), an androgen antagonist, at 1 week of postnatal age (Lee, 2014). Expressional changes of some Cx isoforms are dose-dependent or steroidal hormone-specific but expression patterns of others are not (Lee, 2014). For examples, treatments with ED at 0.015 and 1.5 μg/kg body weight (BW) result in increased expression of Cx30.3 and Cx43. However, expression of Cx31 gene is not affected by 0.015 μg EB/kg BW but is decreased by 1.5 μg EB/ kg BW (Lee, 2014). Expression of Cx43 gene increases with treatments of 500 μg or 50 mg Flu/ kg BW, while transcript levels of Cx32 and Cx37 are only increased by a treatment of 50 mg Flu/ kg BW (Lee, 2014). Together, the findings from our and other researches strongly imply that expressional regulation of Cx gene in the IS is influenced by androgen and/or estrogen in part.
It is generally documented that functions of the epididymis are regulated by the actions of androgen, as well as estrogen (Joseph et al., 2011; Robaire & Hamzeh, 2011). During the early postnatal period, serum testosterone concentrations are not steady, and expressional levels of androgen and estrogen receptors in the epididymis are gradually increased (Sar & Welsch, 2000; You & Sar, 1998). Thus, it is generally considered that responsiveness of the epididymis to androgen and estrogen at different postnatal age would be diverse. Androgen and estrogen chiefly control expression of a number of genes involving in the functional regulation of the epididymis (Joseph et al., 2011; Robaire & Hamzeh, 2011). As mentioned earlier, expression of Cx isoforms in the adult IS are altered by exogenous administration of EB or Flu at 1 week of postnatal age (Lee, 2014). Thus, the present study was designed to determine if hormonal disruption by exposing to EB or Flu at the early or late neonatal age produces similar results in expression of Cx isoforms in the adult IS. Unlike treatment at 1 week of age in the previous research, EB or Flu was exogenously administrated at 3 weeks of age. Expressional patterns of Cx isoforms in the adult IS were analyzed by quantitative real-time polymerase chain reaction.
MATERIALS AND METHODS
Pregnant Spragure Dawley rats (n=5) obtained from Samtako (OSan, Korea) were kept in individual cage upon the arrival and randomly assigned into one of experimental groups, including control, low-dose estradiol benzoate (EB) treated (EB-L), high-dose EB treated (EB-H), low-dose flutamide (Flu-L), and high-dose flutamid (Flu-H) group. Free access to food and drinking water was allowed for the experimental period. Five to seven pups were delivered from each female rat and kept together with mother rat until the weaning at 21 days of age.
The EB and Flu were purchased from Tokyo Chemical Industry Co. (Tokyo, Japan). To prepare EB and Flu stock solutions, the powder of hormones was stirred in 100% EtOH at room temperature until completely dissolved. The stock solutions were diluted in peanut oil to make working solutions. At the weaning, each male pup was weighted, and an amount of steroid hormone being subcutaneously injected was calculated. The final concentrations of EB and Flu were 0.015 μg/kg body weight (BW) for EB-L, 1.5 μg/kg BW for EB-H, 500 μg/kg BW for Flu-L, and 50 mg/ kg BW for Flu-H. Subcutaneous injection of peanut oil of same amount was applied to animals in control group. The doses of EB and Flu were chosen because a previous research had shown that neonatal treatment of these hormones at the doses resulted in recognizable influences on expression of Cx isoforms in the IS (Lee, 2014)
At 4 months of age, experimental animals were anestheticzed by CO2 stunning in a chamber. An incision was made on the lower abdomen to expose the reproductive tract. The epididymis was separated from the testis and epididymal fat in cold PBS. Careful dissection was carried out to isolate the IS from the rest of the epididymis. The IS was washed in fresh cold PBS for two times and frozen in liquid nitrogen. The IS was kept in –80°C until utilized for total RNA isolation and cDNA generation. A total of 30 male rats were used for the present study, including control (n=6), EB-L (n=6), EB-H (n=6), Flu-L (n=6), and Flu-H (n=6).
The frozen IS was homogenized in an Eppendorf tube having total RNA extraction solution (iNtRON Biotech, Sungnam, S.Korea) with a polytron homogenizer (Fisher Scientific, Pittsburgh, PA). Then, a pellet of total RNA was isolated using common phenol-chloroform extraction method. The total RNA in DEPC-dH2O was resuspended in DEPC-dH2O, and the concentration of total RNA was analyzed with an UV spectrophotometer (Eppendorf, New York, USA). In addition, gel electrophoresis was carried out for qualitative evaluation of total RNA samples. The total RNAs were either directly utilized to generate cDNA or stored in –80°C until utilized later for cDNA construction.
One microgram of total RNA was used to generate the first strand of cDNA in ImProm-II™ reverse transcription system (Promega, Madison, USA) with oligo-dT primer. The mixture of reverse transcription (RT) reaction was placed in 25°C for 5 min, 42°C for 1 hr and 30 min, and then 70°C for 15 min. The constructed cDNA was directly utilized for quantitative real-time PCR to evaluate expressional changes of Cx isoforms among experimental groups. Oligonucleotide primers for real-time PCR analysis are described in Table 1.
The mixture for real-time PCR included 1 μL of cDNA, 10 pmol of each primer, 10 μL of master mixture (Finnzymes, Espoo, Finland), and nuclease-free dH2O to make a final volume of 20 μL. The PCR was first placed at 95°C for 30 sec for denaturation of RNA and primer strands. Then the PCR was performed in cycles of denaturation stage at 95°C for 30 sec, annealing stage at Tm for 30 sec, and extension stage at 72°C for 30 sec. An additional extension step at 72°C for 10 min was included at the end of each PCR cycle. Cyclophilin A (Ppia) was included in every PCR for an internal quantitative control purpose. Agarose gel electrophoresis was performed to confirm correct sizes of PCR products.
Of total RNAs isolated from IS tissues, randomly chosen four total RNA samples were used to conduct RT reaction and real-time PCR. Relative expression levels of the Cx genes were calculated according to the 2-ΔΔCt method. Experimental results are present in relative expression ratios between Ppia and Cx genes. One-way ANOVA was carried out to determine statistical significance among control and experimental groups of EB or Flu treatment. Whenever the statistical significance was present, a post-hoc analysis, Duncan’s t test, was applied to find out differences among these experimental groups. A p value < 0.05 was considered statistically significant.
RESULTS
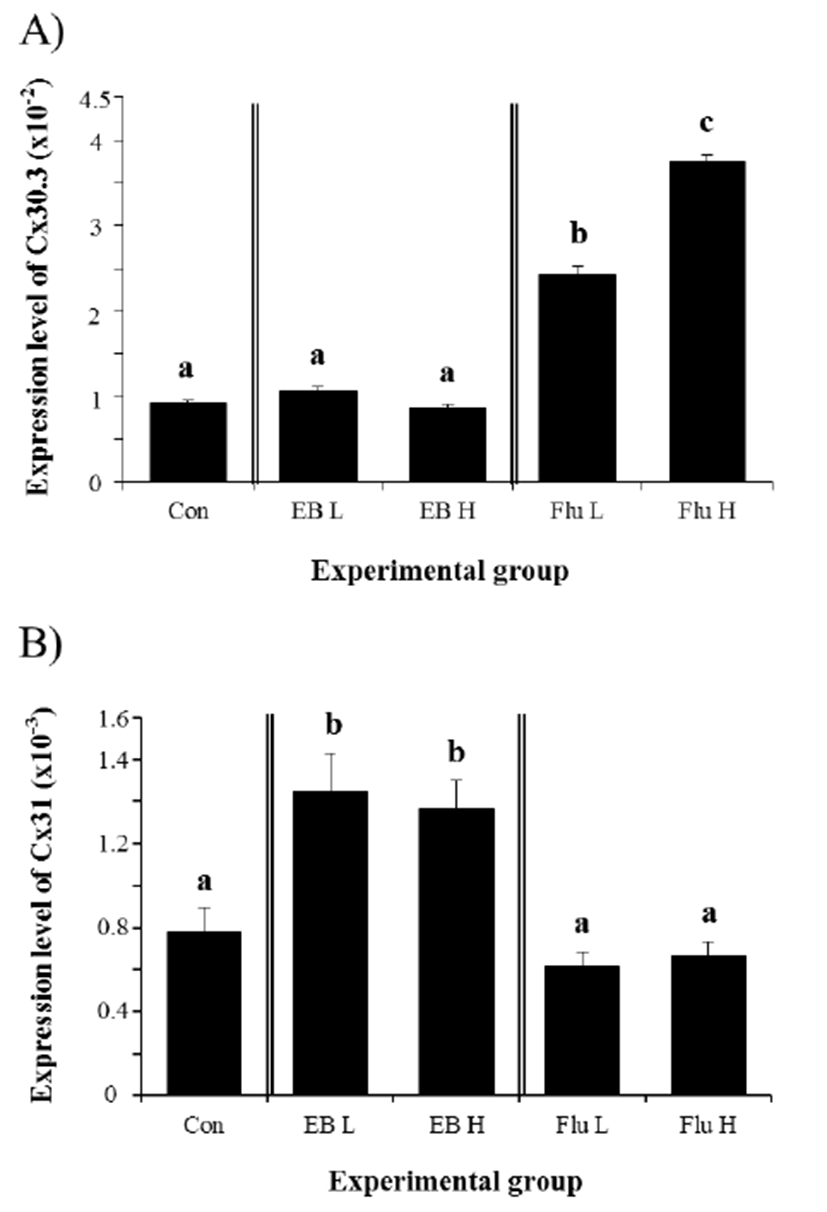
Expression of Cx30.3 in the IS of adult rat was not significantly changed by the treatment of EB at 3 weeks of age (Fig. 1A). Compared with control group, neither low-dose nor high-dose of EB affected the level of gene expression of Cx30.3 in the IS, (Fig. 1A). However, the treatment of Flu at low-dose resulted in a significant increase of Cx30.3 gene expression (Fig. 1A). A further great increase of Cx30.3 expression was induced by high-dose Flu treatment (Fig. 1A). Expressional change of Cx31 gene in the IS was observed with EB treatment (Fig. 1B). Treatment of both doses of EB at 3 weeks of age caused significant increases of Cx31 gene in the adult IS, even though there was no statistical difference on Cx31 transcript level between low-dose or high-dose EB treatment (Fig. 1B). Unlike Cx30.3, the treatment of Flu didn’t give an influence on gene expression of Cx31 in the IS (Fig. 1B).
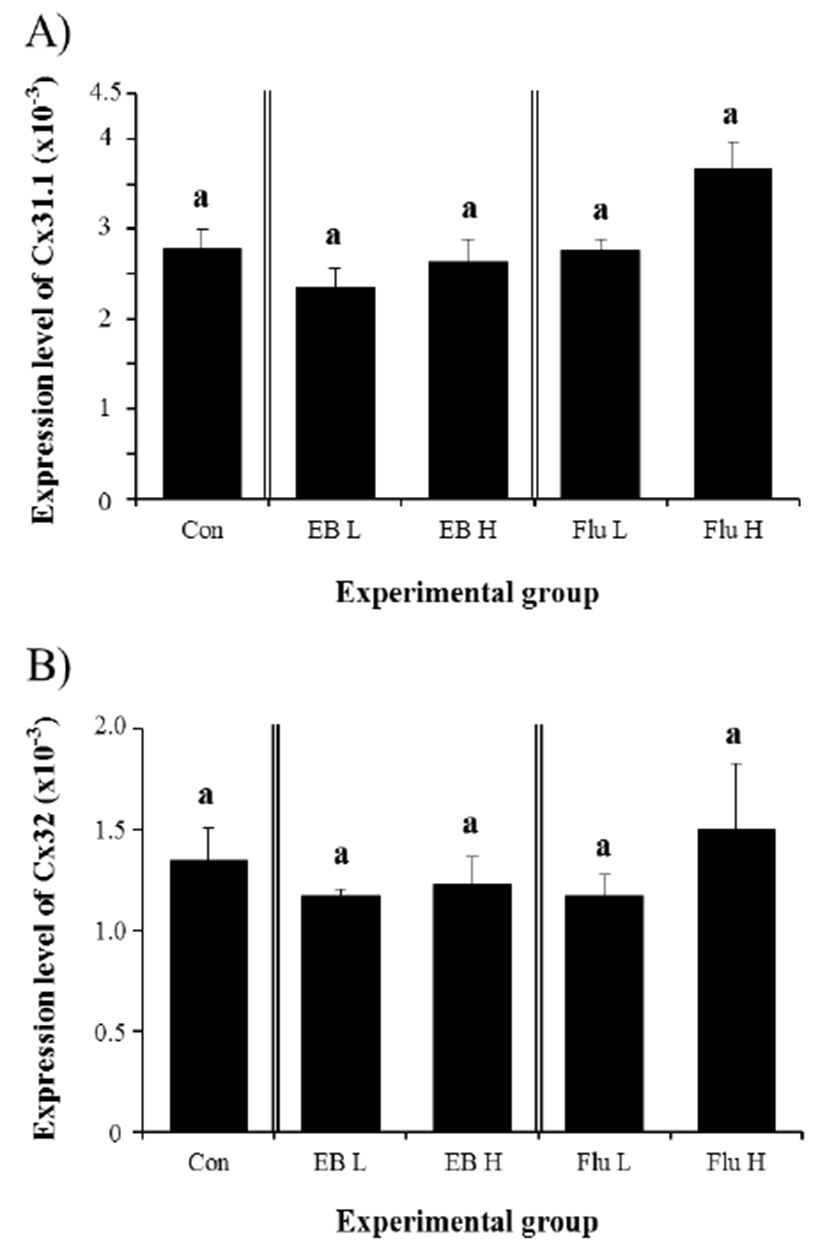
Expression of Cx31.1 gene was not affected in the adult IS by treatment of EB at 3 weeks of age (Fig. 2A). There was no significant statistical change of Cx31.1 transcript levels in both EB-treated groups, compared with that in control group (Fig. 2A). Similarly, the treatment of Flu at 3 weeks of age didn’t give an influence on the expression of Cx31.1 in the adult IS, even though a slight increase of Cx31.1 transcript level, but not statistically significant, was observed with a high-dose Flu treatment (Fig. 2A). Compared with the control, no significant change of Cx32 gene expression was detected in the adult IS treated with EB at 3 weeks of age (Fig. 2B). Also, the treatment of Flu at 3 weeks of age didn’t cause expressional modulation of Cx32 gene in the adult IS (Fig. 2B).
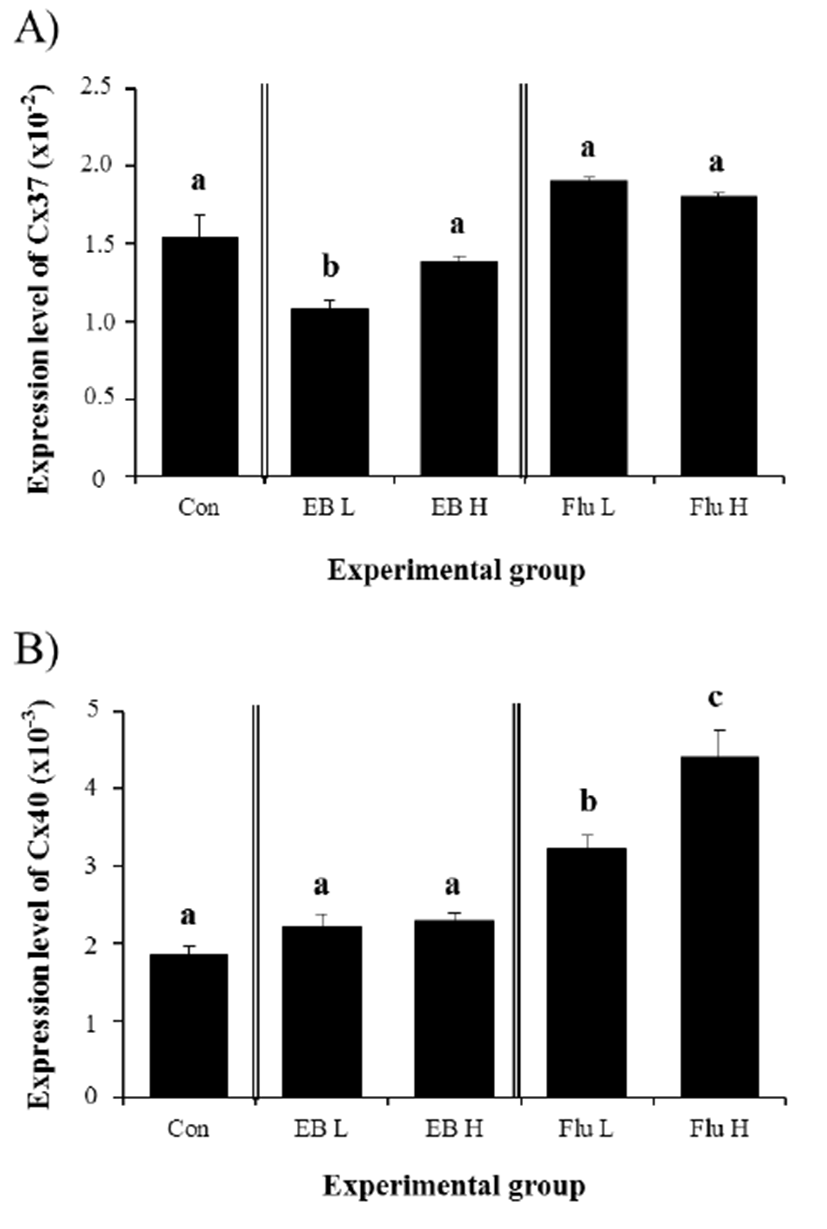
Expression of Cx37 in the IS at the adult was significantly decreased by a low-dose EB treatment at 3 weeks of age (Fig. 3A). However, treatment of EB treatment at a high-dose didn’t give an influence on expressional modulation of Cx37 in the adult IS (Fig. 3A). None of Flu treatments at two different doses at 3 weeks of age caused significant change of Cx37 transcript level in the IS at the adult (Fig. 3A). Regarding expression of Cx40, treatment of EB at two different doses didn’t result in significant expressional change in the adult IS (Fig. 3B). However, a treatment of Flu at a low-dose at 3 weeks of age led to a significant increase of Cx40 transcript level in the adult IS (Fig. 3B). A further statistically significant increase of Cx40 gene expression in the adult IS was observed with a high-dose treatment of Flu at 3 weeks of age (Fig. 3B).
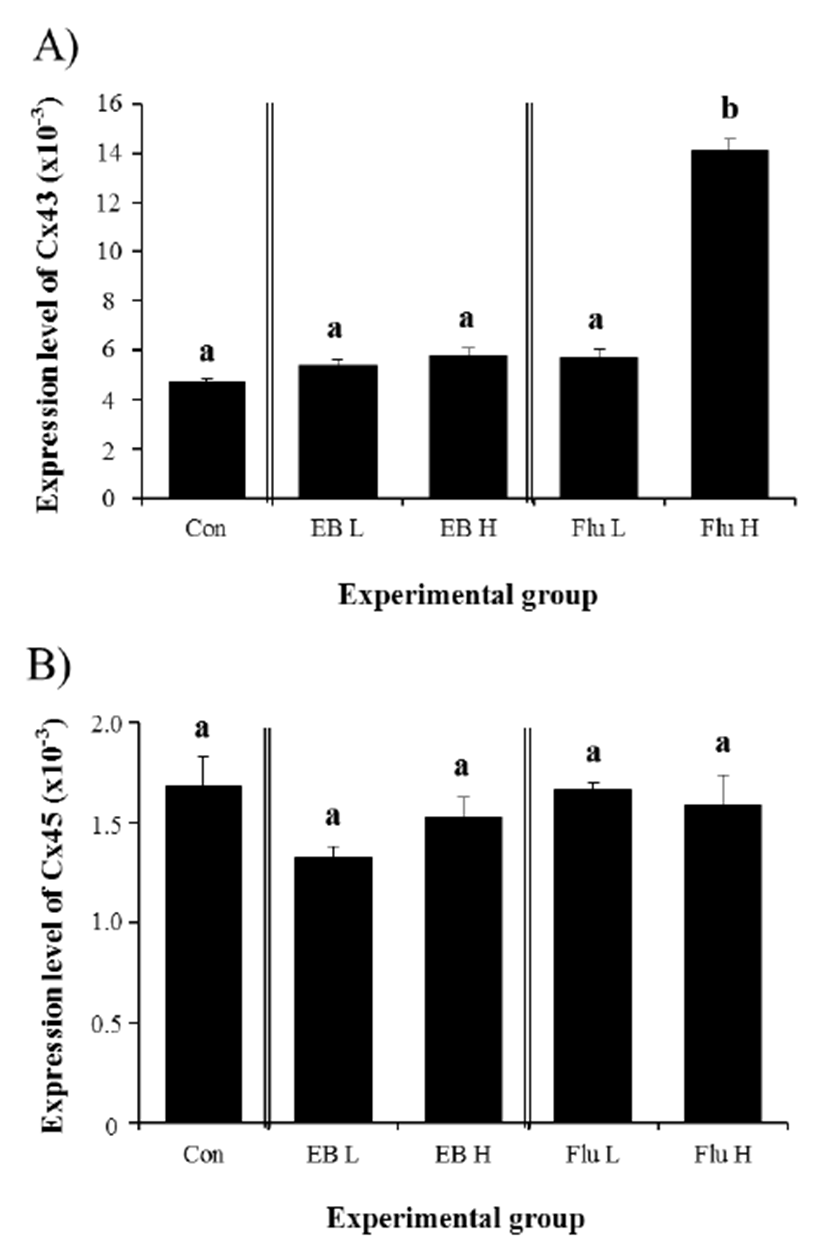
Expression of Cx43 in the IS of the adult was not changed by EB treatment at 3 weeks of age (Fig. 4A). Compared with the control, the transcript level of Cx43 gene in the adult IS treated with different doses of EB at 3 weeks of age was not significantly different (Fig. 4A). Treatment of a low-dose Flu at 3 weeks of age didn’t cause any change on the level of Cx43 transcript in the adult IS (Fig. 4A). However, a significant increase of Cx43 expression in the IS of the adult was detected with a treatment of Flu at a high-dose at 3 weeks of age (Fig. 4A). Even though mean levels of Cx45 transcript in all experimental groups were lower than that in control group, transcript levels of Cx45 in EB- and Flu-treated experimental groups were not significantly different with that of control group (Fig. 4B).
DISCUSSION
Exogenous exposure to steroidal compounds during the early developmental period commonly accompanies disruption of hormonal homeostasis and gene expression in target tissues. Main concern of the present research was to examine the effect of steroidal agonist or antagonist treatment at the late neonatal age on the gene expression of Cx isoforms in the adult IS. Experimental results showed that consequences of administration of EB or Flu at 3 weeks of age were aberrant expression of some Cx isoforms, but not all, in the adult IS.
The most notable results from the present study are that expression of Cx31.1, 32, and 45 genes in the adult IS was not affected by EB or Flu treatment at 3 weeks of age. Transcript levels of Cx31.1 in the adult IS were significantly changed by treatment of low-dose EB or Flu to male rat at 1 week of age (Lee, 2014). The treatment of EB or Flu at 1 week of age caused expressional modifications of Cx32 and 45 after EB or Flu in the adult IS (Lee, 2014). A difference between the previous and current researches was only the age of experimental animal used. Even though the epididymal epithelium shares common cell types, such as principal and basal cells, the epithelium of IS possesses specific cell types, including apical and narrow cells (Robaire & Hermo, 1988). Appearance of these cell types in the epithelium of epididymis is developmentally regulated. That is, until the first week after the birth, there is no obvious differentiation of the columnar epithelial cells in the IS (Robaire et al., 2000). The epithelial differentiation of the epididymis begins at the end of the first week after the birth, and completed at the puberty (Robaire et al., 2000). These reports indicate that the epithelium of IS at different postnatal age, especially during neonatal period, is likely organized with different cell types, and cellular composition of these cell types would vary during the early postnatal development. Moreover, expression of androgen receptor in the epithelium of epididymis during the early postnatal period is increased over time (You & Sar, 1998), and the epithelium of IS possesses functional estrogen receptor (Cooke et al., 1991). Together, it would be reasonable to consider that responsiveness and sensitivity of the IS to estrogen and androgen vary with age during postnatal development, especially during neonatal period. These events could lead to differential expression of diverse molecules associated with differentiation and maturation of epithelial cells, thereby functional development of the IS. Based on this speculation, different expression patterns of Cx31.1, 32, and 45 between the present research and a previous study (Lee, 2014) would result from different response to treatments of an estrogenic compound, EB, or an antiandrogenic compound, Flu, at different postnatal age, at least. Additional experiments conducted at other neonatal ages could provide more solid evidence on this assumption.
In the present study, significant increases of Cx30.3 transcript level are only detected with Flu treatment, while treatments of EB or Flu of a high dose at 1 week of age result in significant increases of Cx30.3 expression in the adult IS (Lee, 2014). Moreover, the previous research has shown significant a decrease of Cx31 expression in the adult IS by treatment of EB of a high dose or Flu of a low dose at 1 week of age (Lee, 2014). However, expression of Cx31 is significantly increased with EB treatment at 3 weeks of age. Such disagreements of results on gene expression would be due to differential responsiveness to steroid molecules exposed exogenously in the IS at different postnatal ages.
Treatments of EB or Flu at different neonatal ages also result in differential expression patterns of Cx37, 40, and 43 in the adult IS. Significant changes of Cx37 transcript levels are detected with Flu treatment at 1 week of age (Lee, 2014), while a treatment of low-dose EB at 3 weeks of age only results in a decrease of Cx37 expression. A significant increase of Cx40 expression in the adult IS is detected with high-dose Flu treatment at 1 week of age (Lee, 2014), even though treatments of low- and high-dose Flu at 3 weeks of age lead to increases of Cx40 transcript level in the adult, as observed in the present research. Moreover, expression of Cx43 gene is significantly increased with EB or Flu treatment at 1 week of age (Lee, 2014), but only high-dose Flu treatment at 3 weeks of age induces a great increase of Cx43 transcript level. It is difficult to conclude how estrogen and/or testosterone influence on expression of Cxs in the IS at this point due to limited information available on regulation of individual Cx gene expression. Cry et al. (1996) have suggested expressional regulation of Cx43 in the adult epididymis by androgen. However, because this research was carried out in the adult experimental animals, the effect of androgen on Cx43 gene expression could not be directly applied to the findings from the present study. Future researches are required to determine a role of steroid hormones on gene expression of Cx genes in the IS during the early postnatal period.
In conclusion, it is clear that expression of Cx isoforms in the IS during the neonatal period is differentially regulated by estrogenic and androgenic molecules. However, it seems that transcriptional regulation of each Cx gene is controlled by these steroid hormones in dose- and/or age-specific manners. The present study shows a possibility of altered expression of Cx genes in the adult IS by exogenous exposure to steroidal substances at the early postnatal development.

