INTRODUCTION
Thyroid hormones (THs) are vital for the maintenance of normal physiology in humans and animals. Hypothyroidism is a disorder of the endocrine system in which the thyroid does not produce enough THs, affecting up to 5% of the general population with a further estimated 5% being undiagnosed in USA (Chiovato et al., 2019). It can cause a wide range of symptoms, such as poor ability to tolerate cold, a feeling of tiredness, constipation, slow heart rate, depression, and weight gain (El-Shafie, 2003; Idris et al., 2005).
In older children and adolescents, the symptoms of hypothyroidism are similar to those in adult, and particularly, include irregular menstrual cycles and delayed puberty in girls (Anasti et al., 1995; Counts and Varma, 2009). In hypothyroid rodents, however, the delay of puberty onset in female is uncertain while it is prominent in male (Wilen et al., 1981; Shibutani et al., 2009). So far, this discrepancy has not been investigated in detail.
6-n-propyl-2-thiouracil (PTU) is a medication used to treat hyperthyroidism, also is widely applied to induce hypothyroidism in experimental rodents (Mandel and Cooper, 2001; Mallela et al., 2014). The present study was performed to understand the relationship between maternal hypothyroidism and puberty onset in female rat offspring. To do this, we employed PTU-induced hypothyroid rat model. Based on our pilot studies, we used slightly modified PTU model (Hapon et al., 2007; Zamoner et al., 2008; Tanaka et al., 2019). Comparison of general indices such as body and tissue weights, and puberty indices such as vaginal opening (VO) and tissue histology between control and PTU-treated rats were conducted.
MATERIAL & METHODS
6-Proryl-2-thiouracil (PTU; CAS no. 51-52-5, purity>99.0%) was purchased from Tokyo chemical industry (Tokyo, Japan). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pregnant Sprague–Dawley rats were purchased from DBL (Eumseong, Korea) at gestational day (GD) 12. The animal rooms were maintained in air-conditioned temperature 22±1°C, relative humidity 40±5% with 12-hour light/dark cycle. Dam and offspring were fed ad libitum with Purina Mouse Diets, purchased from Biopia (Gunpo, Korea). The animal protocols were approved by the Animal Care and Use Committees at Sangmyung University (approval number R-2001-1). All the animals received humane care in accordance with the guides for animal experiments of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Dams were given drinking water containing 0.025% of PTU w/v or water alone from GD14 to at postnatal day (PND) 21 as previously reports with slightly modification, for longer exposure period and lower dose than original procedures (Hapon et al., 2007; Zamoner et al., 2008). The start date of PTU treatment was determined to be the 13th day of gestation (GD13) according to the report that thyroid hormone receptors were first expressed in the fetus on the GD14 of pregnancy (Dowling et al., 2000). PTU containing water was freshly changed every two days. Pups of control group were weaned at PND 21, and dams in PTU-exposed group were further maintained for additional 7 days without PTU-treatment for further nursing of offspring showing severe growth suppression due to hypothyroidism (Tanaka et al., 2019). Body weights and the dates of VO were measured until PND 36. Animals were sacrificed, and the tissue weights were measured between 18:00–19:00 pm. The tissues were fixed in paraformaldehyde (4%) for histological study.
Fixed tissues (thyroids, ovaries, uteri and ovarian fat fads) were dehydrated in graded concentrations of ethanol (70%, 80%, 90%, 95%, and 100%; Duksan, Korea) for 1 hour 30 minutes in each with gentle shaking and soaked in absolute ethanol overnight. The tissues were immersed in xylene (Samchun Chemical, Seoul, Korea ) for 30 minutes, 3 times and in paraffin (Avantik Biogroup, Pine Brook, NJ, USA) at 56°C for 30 minutes, 3 times. The tissues were embedded in paraffin and sectioned (Microm, Walldorf, Germany) at 5 μm. The samples were attached on microscope slides (Marienfeld, Lauda-Königshofen, Germany) and the slides were stained with hematoxylin (Sigma-Aldrich) and eosin (Across, Carson, CA, USA) for 5 minutes, respectively. The width of the thyroid follicle lumen was measured using Image-J software, and other histological parameters such as thickness of the follicular cells were measured.
RESULTS
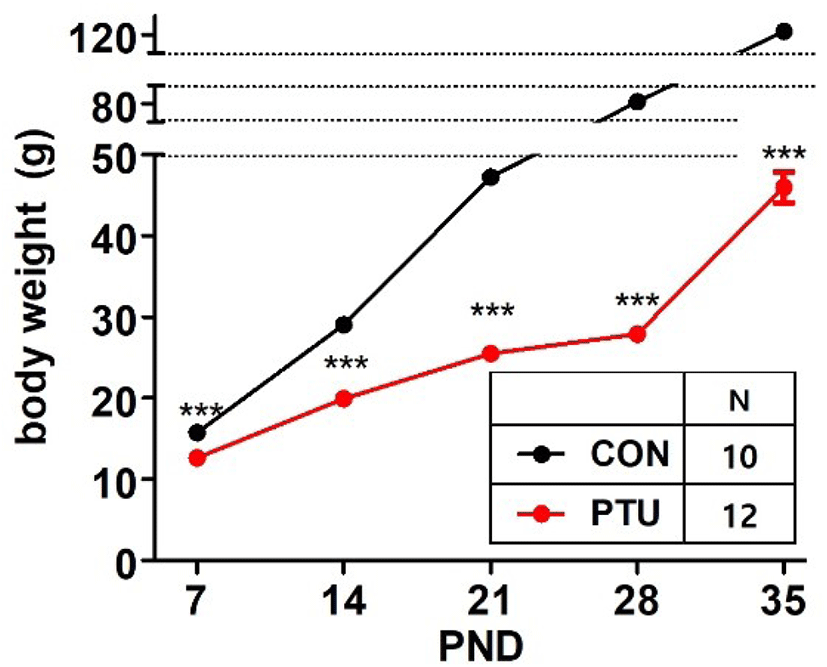
Fig. 1 shows that maternal PTU administration significantly inhibited weight gain in hypothyroid pups (p<0.001). The offspring weights of two groups differed significantly from PND7. The gap in average weight continued to increase thereafter until PND 35.
There was no significant difference in the date of VO, which is a hallmark of the puberty onset, between control and PTU group (Fig. 2). In both groups, VO of the most objects were observed at PND36, but some animals of PTU group had checked relatively later.
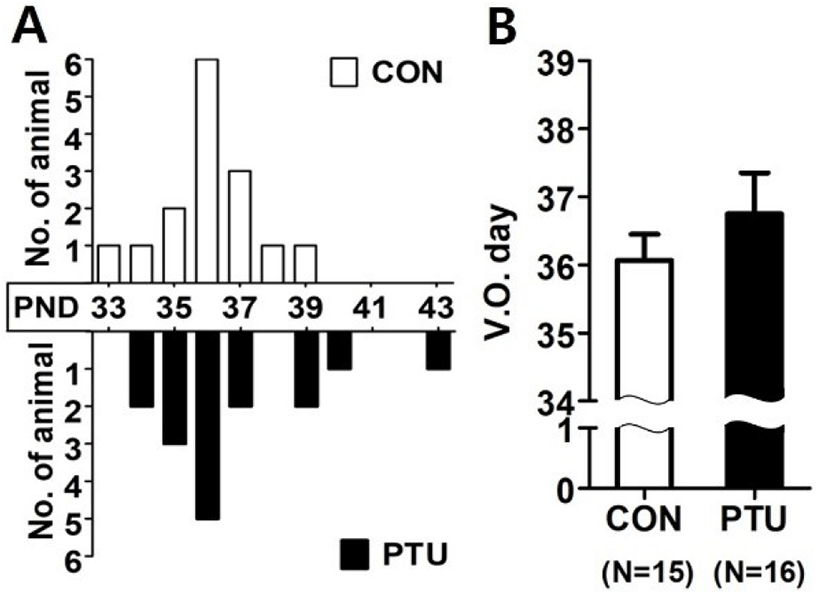
The average body weight at the time of VO showed a huge difference between the two groups. The body weights of the PTU group were significantly lower, only 36.8% of the control group (p<0.001, Table 1).
| CON | PTU | |
|---|---|---|
| Body weight (g) | 138.0±3.69 | 50.8±3.15*** |
Values are expressed as mean±SE. CON (n=12), PTU (n=13).
Differences between groups were considered as significant at p<0.05 and analyzed with Mann-Whitney test (*** p<0.001).
PTU, propylthiouracil.
Fig. 3 demonstrated the estrous cyclicity observed after VO. Each stage of the cycle was determined by the predominant presence of either lymphocytes, nucleated epithelial cells, or keratinocytes (see figure legend for details). Serial microphotographs of the smeared cells from control animals revealed the good cyclicity while no cyclicity was found in the cells from PTU-treated animals (Fig. 3A). The normal estrous cycle has a characteristic periodicity, with an estrus every 4–5 days. The representative patterns of vaginal smears for 10 days post VO also showed the four stages in control animals, while only one persistent stage was found in PTU-treated animals (Fig. 3B and C, respectively).
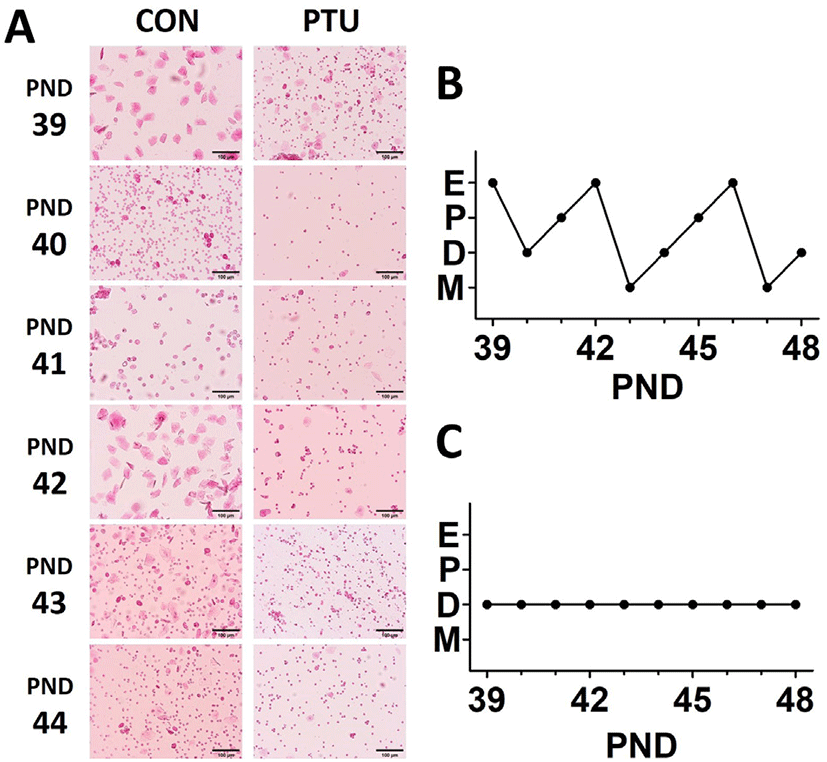
Table 2 showed the effect of PTU administration on changes in tissue weight at VO day. The absolute weight of all tissues except for the thyroid gland decreased significantly, and the relative weights increased significantly except for the ovary and uterus (Table 2). Although the absolute thyroid weight was not changed by PTU treatment, the relative weight increased significantly about 2.8 times (p<0.001), indicating that the hypothyroidism pursued by this experimental model was successfully induced. On the other hand, the absolute weights of the ovary and uterus were markedly decreased by PTU administration (p<0.001), the relative weights were not significantly changed. This phenomenon is interesting, because it suggests that the pubertal growth of thyroid gland might be proceeded regardless of somatic growth, but not in other organs.
Relative tissue weight calculations are as follows, depending on the tissue.
Thyroid, ovary, adrenal: relative tissue weight=[Tissue weight (mg)/Bodyweight (g)]×100.
Uterus, kidney, spleen: relative tissue weight=[Tissue weight (mg)/Bodyweight (g)]×10.
Values are expressed as mean±SE Groups marked with an asterisk are significantly different from control group, * p<0.05, ** p<0.01, *** p<0.001.
PTU, propylthiouracil.
At PND 36, when VO was not checked in control while VO was recorded in PTU animal, tissue samplings for histological study were performed. Although the absolute weights of the thyroid gland of both control and PTU group were similar (Table 2), histological analysis revealed that the area of thyroidal follicular lumen and the width of follicular epithelial cells were greatly reduced in the PTU group. As expected, the size of the ovaries was markedly reduced in PTU group (Table 2). There were many primary and secondary follicles and no corpus luteum in the control group, but relatively few primary and secondary follicles and many tertiary follicles and corpus luteum-like structures were found in the PTU group (Fig. 4). The size of the uterus was relatively small in the PTU group (Table 2), however, the endometrium, lumen, and muscle layers of the PTU group were underdeveloped states compared to those of control group. In case of ovarian fat pad, more cell nuclei and reductions in cell area were observed in the PTU group.
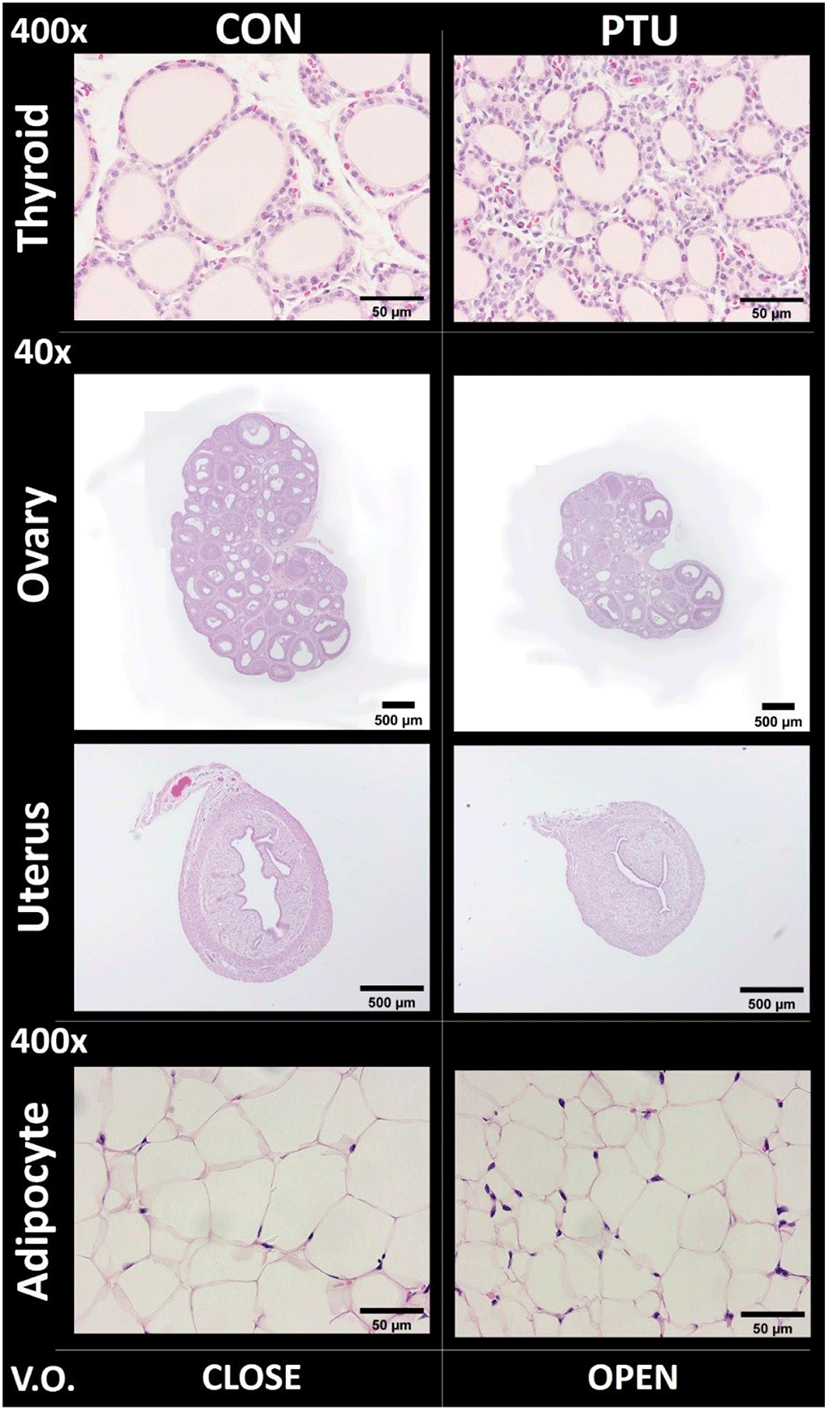
Table 3 showed histological parameters such were luminal area, epithelial cell width, gland number, follicle number, and adipocyte cell number. In the thyroid of PTU group, follicle luminal and epithelial width were significantly reduced (p<0.001). In the uterus of PTU group, widths of perimetrium and myometrium, and epithelial area were significantly reduced (p<0.01 and p<0.001, respectively). In the ovary of PTU group, numbers of primary, secondary and Graafian folicles were not significantly changed, while the number of the corpus luteum was 2.33±2.333 in PTU group and 0 in CON group. In the ovarian fat pad adipocytes of PTU group, cell areas were significantly reduced (p<0.001).
Mother rats were supplied with water or 0.025% propylthiouracil from gestation day 14 to offspring postnatal day (PND) 21. After PND 21, offspring rats were supplied with non-propylthiouracil water until sacrifice. The parameters were measured by ImageJ software.
Values are shown as mean±SE.
Significant differences are marked with asterisks (* p<0.05; ** p<0.01; *** p<0.001; compared with the CON group).
PTU, propylthiouracil.
DISCUSSION
The primary function of the thyroid is the production of the iodine containing THs, triiodothyronine (T3) and thyroxine (T4) and the peptide hormone calcitonin (Hall and Guyton, 2011). THs have a wide range of effects on the human body in metabolism, cardiovascular function, development and play a role in maintaining normal sexual function (Colledge et al., 2010). Two main TH-associated thyroid disorders are hyperthyroidism and hypothyroidism; hyperthyroidism is characterized by excessive secretion of THs, and hypothyroidism is characterized by a deficient secretion of THs (Taylor et al., 2018). In the present study, the absolute weight of the thyroid from female offspring was not changed by PTU treatment from GD14 through PND 21. However, the relative weight was increased significantly about 2.8 times (p<0.001), indicating that the hypothyroidism pursued by this experimental model was successfully induced.
Pituitary TSH, hypothalamic TRH, and thyroid THs form the hypothalamic-pituitary-thyroid hormonal axis (HPT), and in general, elevated blood levels of THs inhibit the release of TRH and TSH, whereas the opposite effect occurs when serum TH levels decrease (Choksi et al., 2003). Furthermore, HPT and the hypothalamic-pituitary-gonadal axis (HPG) are physiologically related and act together as a unified system in some pathological conditions (Doufas and Mastorakos, 2000). There are several reports that speculate how thyroid disorders induce abnormal puberty based on interaction between the two neuroendocrine axes (Castañeda Cortés et al., 2014; Fekete and Lechan, 2014; Kiyohara et al., 2017 ). The junction of the two axes is estimated to be hypothalamic gonadotropin-inhibitory hormone (GnIH, also known as RF amide-related peptide coded by the NPVF gene); TH-mediated HPG regulation may be initiated by decrease of GnIH expression, which acts at the most upstream level of the HPG axis by inhibiting the activity of GnRH neurons to reduce circulating levels of gonadotropins (LH and FSH) and gonadal sex steroids (Tsutsui et al., 2018). The precise mechanism of TH action, however, on puberty onset is still unclear. In addition, there is conflicting results regarding the effect of abnormal thyroid status on reproductive development. For example, hypothyroidism in girls can cause alterations in the pubertal process, usually a delay, but occasionally it can result in pseudo-precocious puberty (Doufas and Mastorakos, 2000). In the present study, we found that there was no significant difference in the date of VO between control and PTU group. It is intriguing that, the body weight and ovarian weight of PTU group were greatly reduced compared to the weights of control group while VO could be checked almost simultaneously in both groups. The ovary of PTU group might have equivalent ability to induce VO, despite of the tiny size. At this time, we tentatively concluded that there is no strong correlation between body/ovarian weights and VO when maternal hypothyroidism model is employed. To confirm this idea, one should carefully compare the available data from several precocious and/ or delayed puberty of rat models (Lee et al., 2009; Sun et al., 2021).
Regarding the determination of the puberty onset in female rodents, date of VO has been accepted as most reliable phenotypic signal. We often found that incomplete opening of vagina still collection of epithelial cells is possible particularly in PTU group, and these cases could make a serious error. Therefore, some auxiliary index will be helpful, and serum hormone levels and histology might be served as final confirming index at postmortem.
Taken together, the present study demonstrates the maternal hypothyroidism induced by PTU administration exerts minimal effect on pubertal development symbolized by VO despite of huge retardation in somatic growth. Further studies using more sophisticatedly designed hypothyroidism model will be especially important, and it will help to advance our understanding of pubertal development and related disorders where and when HPT and HPG axis are mutually controlled.

